Electrostatics: Charging by Conduction, Induction, and Friction
Electrostatics involves forces between charged objects. Learn about charging by conduction, induction, and friction along with an intro to electrostatics.
What is electrostatics?
Electrostatics is the physics that deals with the interactions of static (non-moving) charges.
- Positive charges attract negative charges and repel other positive charges
- Negative charges attracts positive charges and repel other negative charges
In a later unit, we will study current which relates to phenomena created by moving charges.
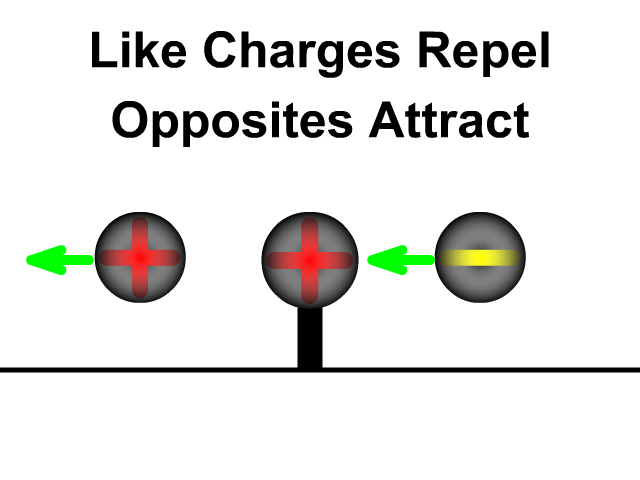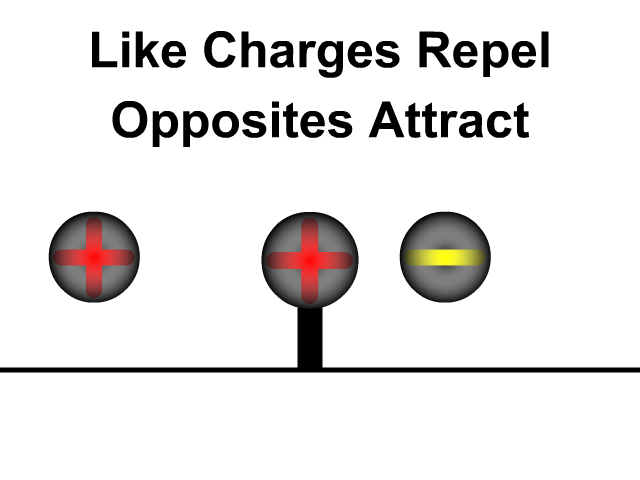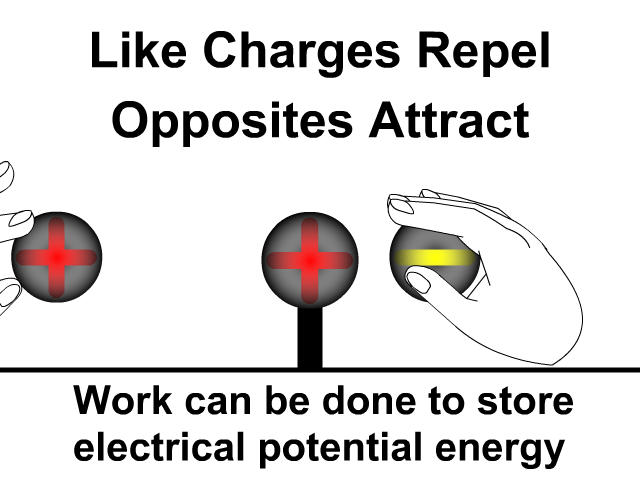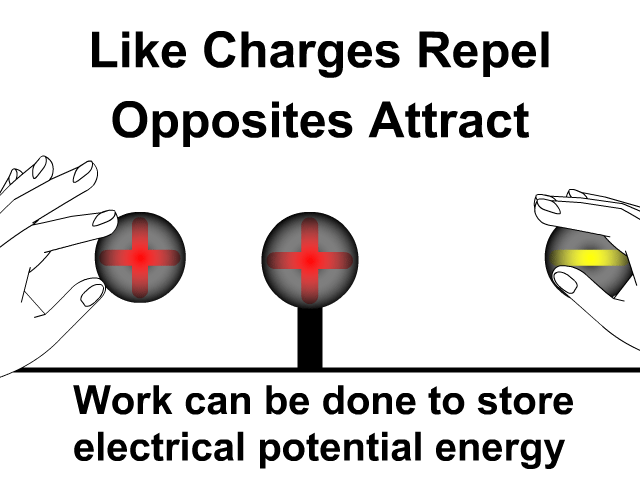
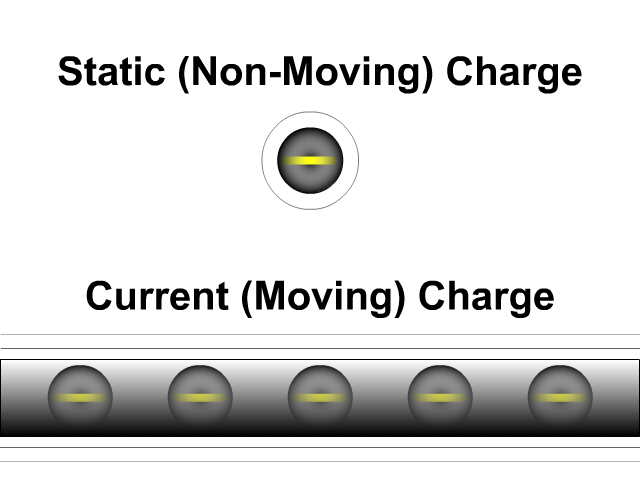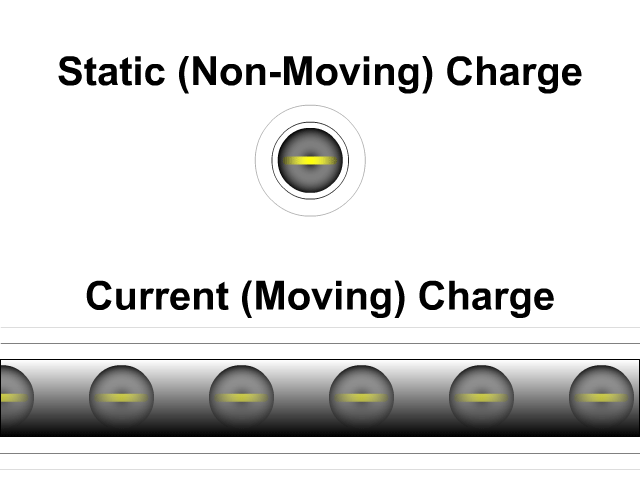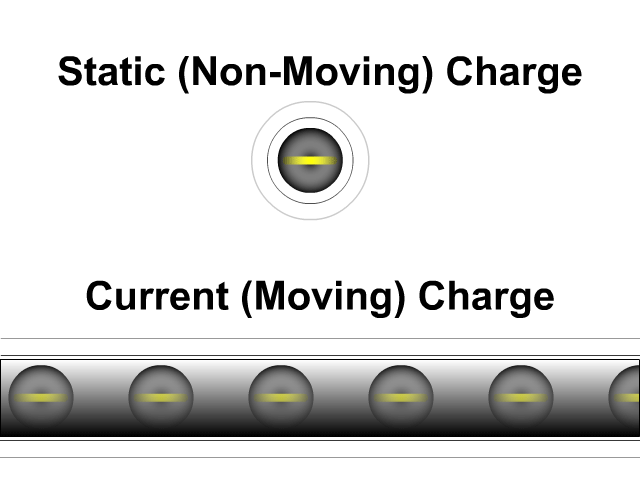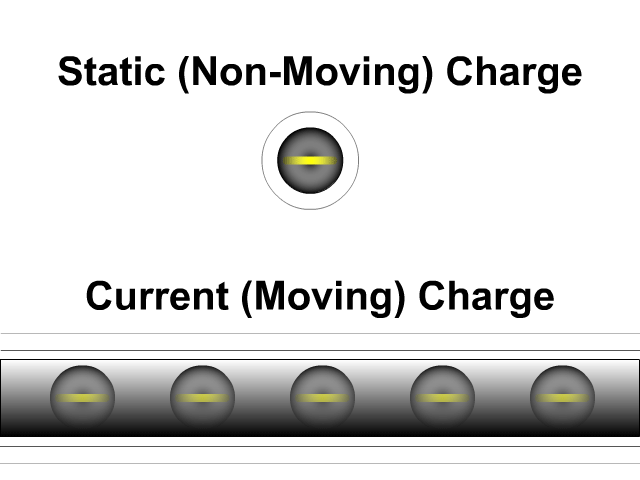
What is the difference between static electricity and current?
Static electricity involves charged objects that are static, which means not moving. Static charged objects create an electric field that interacts with other charged objects around it. When there is a consistent supply of electrons (negative terminal) and another area for those electrons to flow (positive terminal) you get a current. A current is a flow of electrons. They don't flow as directly as our animation here but that is a lesson for a future unit. While a static charge creates a electric field a moving charge or current creates a magnetic field around it.
- Static (Non-Moving) Charges Creates an Electric Field
- Current (Moving) Charges Create a Magnetic Field
Lightning is the result of clouds charging and positive and negative strike lightning is a result of grounding that charge. Learn more about lightning here.
The Atom
Atoms are composed of a nucleus and electron orbits which are regions electrons will most likely occupy. The following table shows the mass and charge of a proton, neutron, and electron.
| Masses and Charges of Subatomic Particles | ||
| Mass | Charge | |
| Proton | 1.673x10-27 kg | +1.602 x 10-19 C |
| Neutron | 1.675x10-27 kg | 0 C |
| Electron | 9.109x10-31 kg | +1.602 x 10-19 C |
In the nucleus you find protons with a positive charge and neutrons that are neutral (no charge). The nucleus is held together by strong nuclear forces.
Every atom comes with one negatively charged electron for every proton that balances the charge of the nucleus. Electrons can be shared through covalent bonds with other atoms to become stable. A stable atom has its outer election shell "or energy level for a defined number of electrons" filled.
The Periodic Table
The periodic table can be referenced to determine how many protons and electrons an atom has. This can be used to determine if it is stable, how reactive it is, and what it will react with to become stable. For this unit the is is important to note that electrons move around readily while protons do not. When an object becomes negative it has gained more negative electrons than protons. When an object becomes positive is has lost electrons. For every object that gained electrons and became negative another lost them and is equally charged positive.
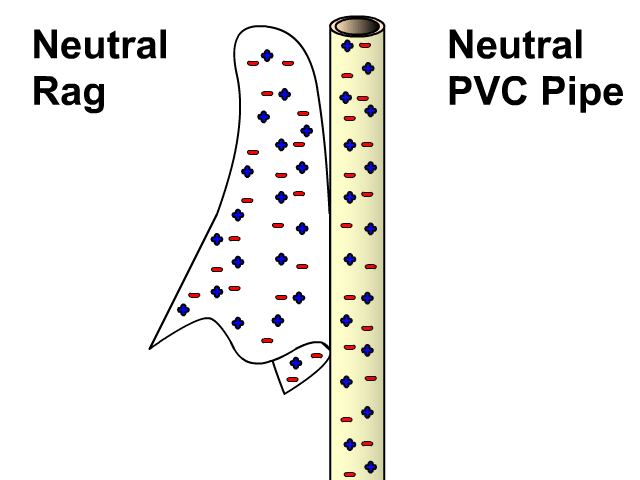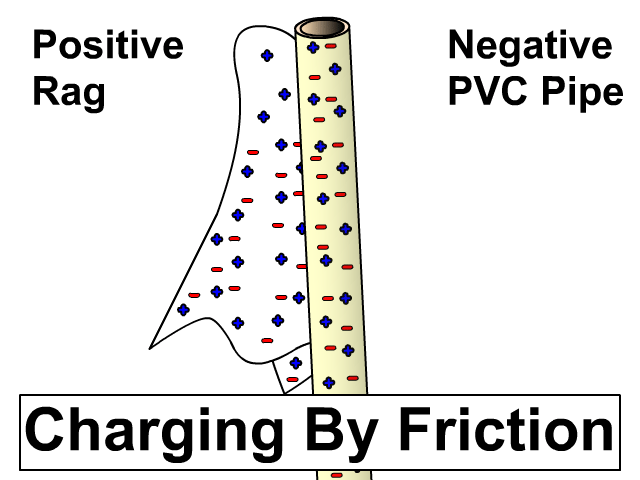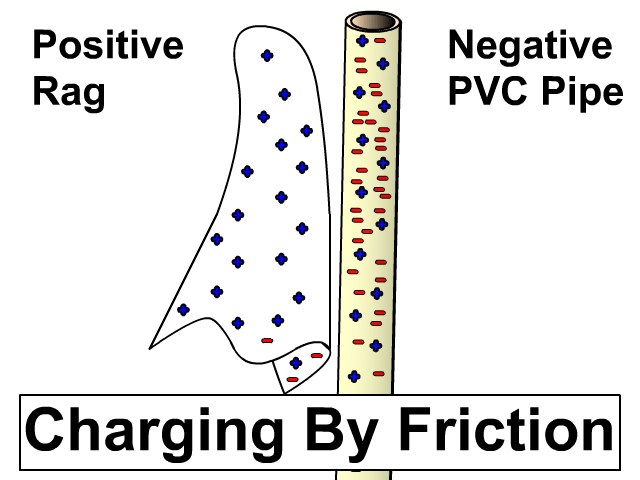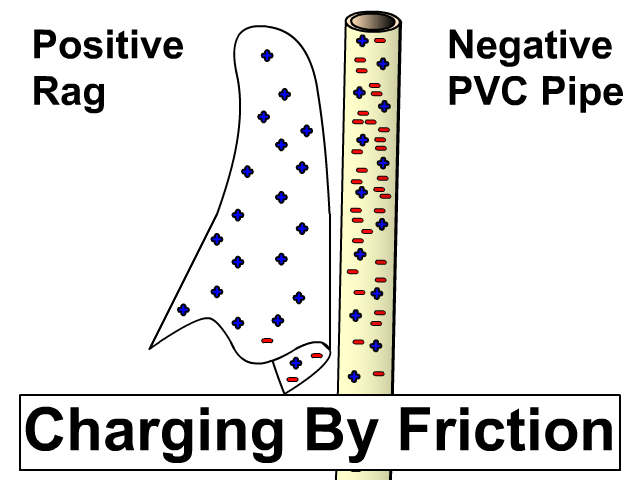
Charging By Friction
Different materials have a different affinity for electrons. Some hold on to their electrons tighter than others.
- Greater affinity for electrons: hold electrons stronger and often gain electrons by friction and become negative.
- Less affinity for electrons: hold electrons weaker and often loose more electrons by friction and become positive.
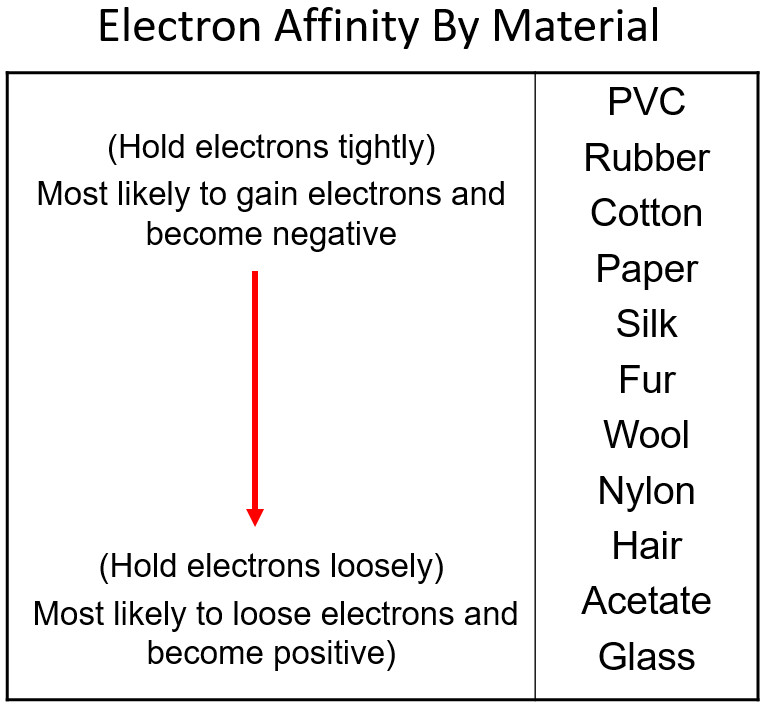
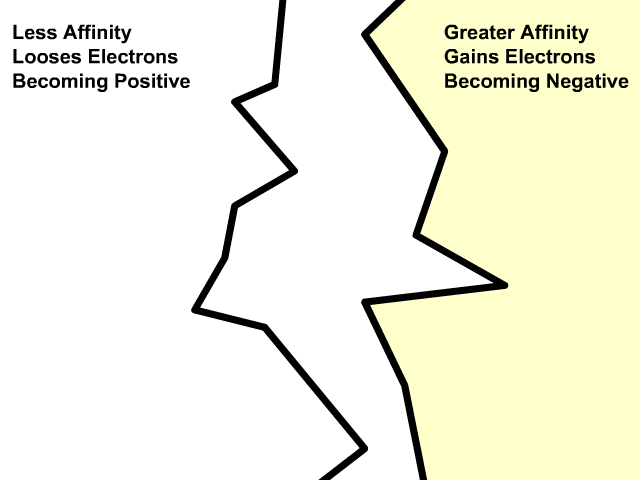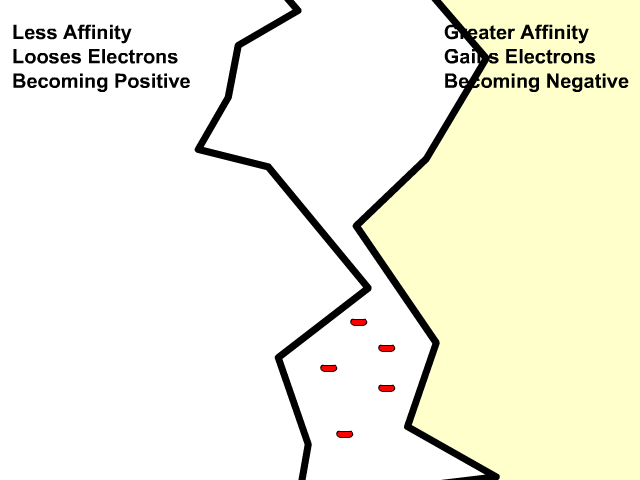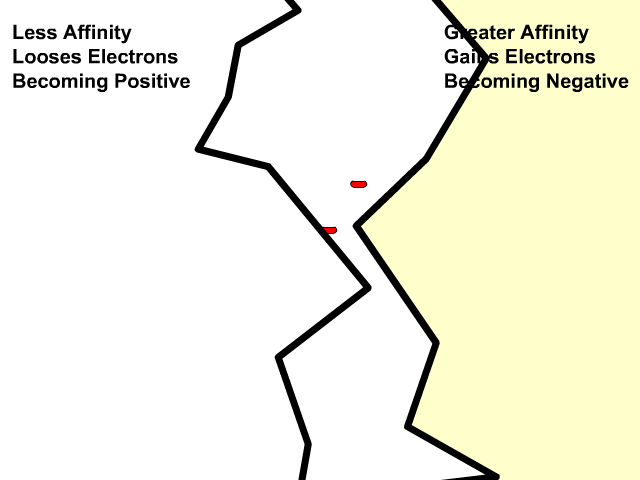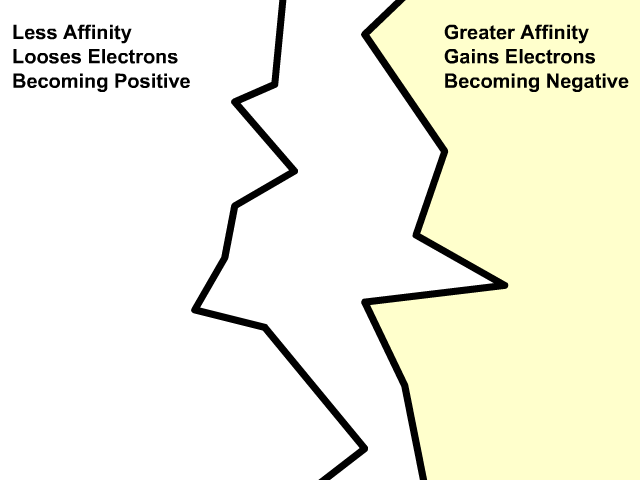
Electron Charge and Electron Affinity
Objects will less affinity for electrons will loose electrons to ones with greater affinity when rubbed together. Friction causes electrons to get bumped out of place with the object that holds them stronger gaining more. The more electrons gained, the more negative the charge. The object that lost those electrons will be equally positive. The net of the two objects will still be neutral. An electron has a charge of -1.6 x 10-19 Coulombs. You will use this value when problems give you a number of electrons and rather that a charge in an electrostatics problem.
- Charge of One Electron: -1.6 x 10-19 Coulombs
- Charge of One Proton: +1.6 x 10-19 Coulombs
Example Problem
You comb your hair on a dry day. The comb gained 2.3 x 104 electrons. Each electron has a charge of -1.6 x 10-19 Coulombs.
1. Does your hair or plastic comb have a greater affinity for electrons? Why?
2. Assume your hair and comb were all neutral before combing. What is the charge of the comb afterwards?
3. What is the charge of your hair afterwards?
Electrical Conductors
Electrical conductors are materials through which electrons move freely.
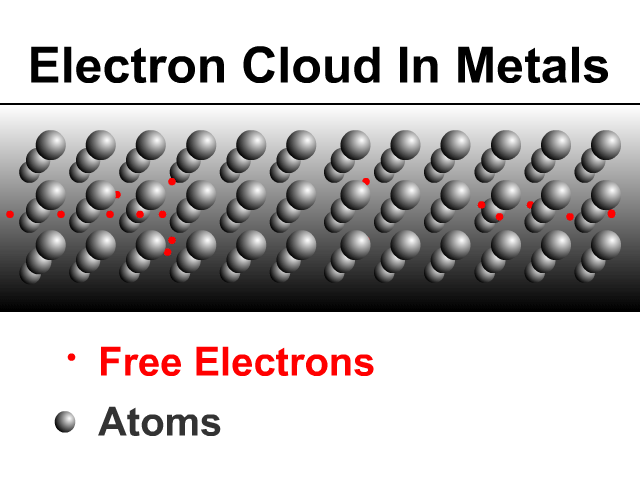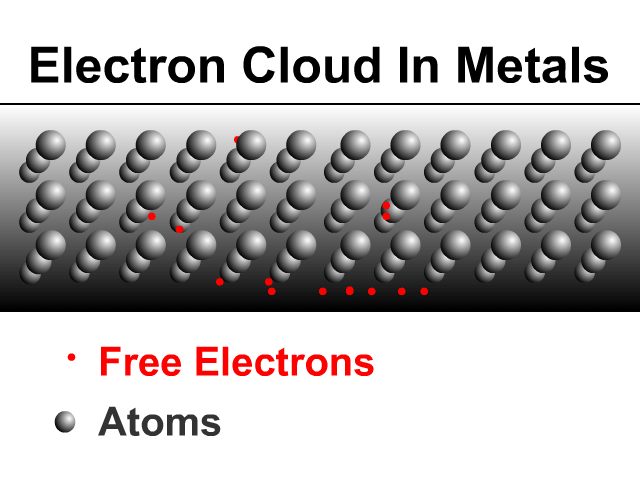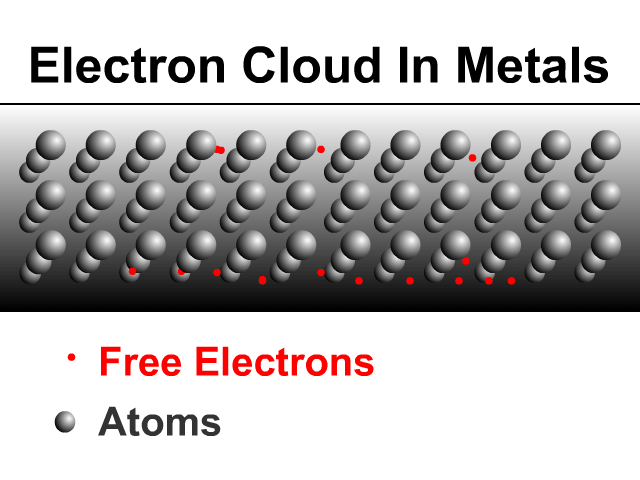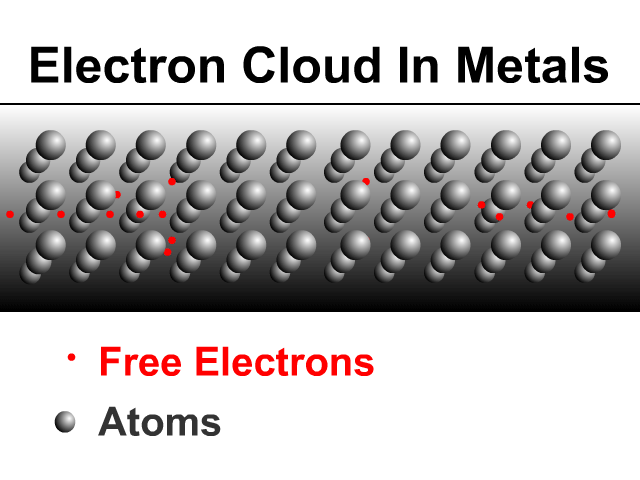
Why are metals good conductors?
Metals are good conductors because of how they share electrons. Metals have a framework of atoms with some loosely held electrons that zip around shared by the metal. This is what many physicists call an electron cloud as seen in the animation.
Top Conductive Metal Elements
- Silver
- Copper
- Gold
- Aluminum
Sharing Charge Between Similar Conductors
When two similar conductors come in contact, electrons separate as much as possible. The net effect is that the two similar conductors will share the original charge. Observe how charge would be shared in our unique animation. Object one shares its charge with initially neutral object two after contact. Now charged, object two continues ahead and equally shares its charge with the next once neutral object. Each time there are two objects sharing charge so the previous charge was divided by two.
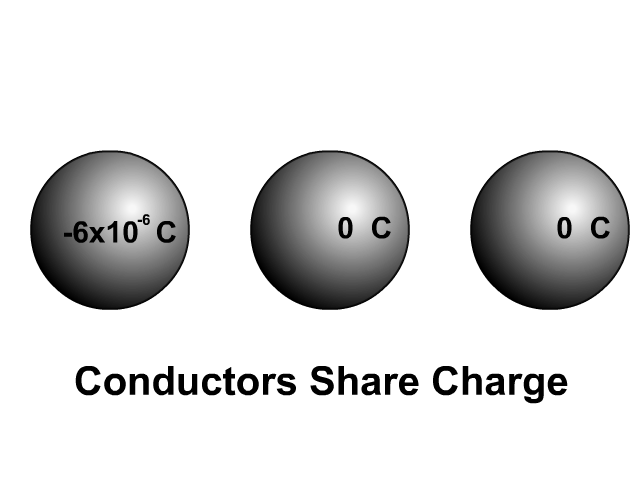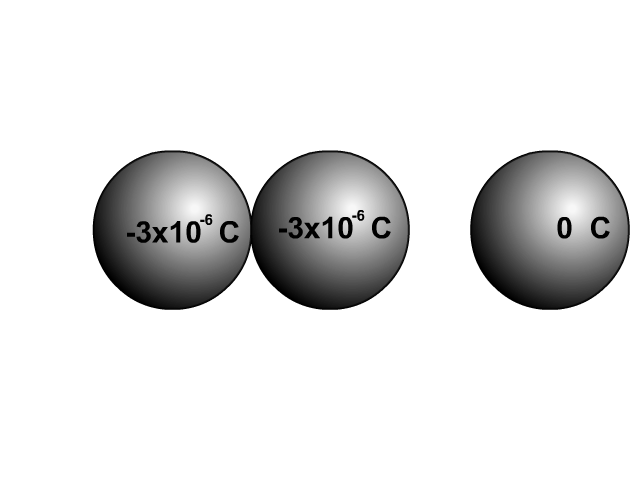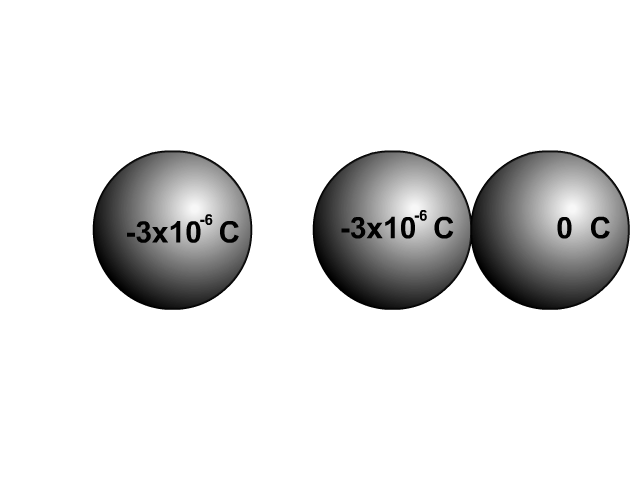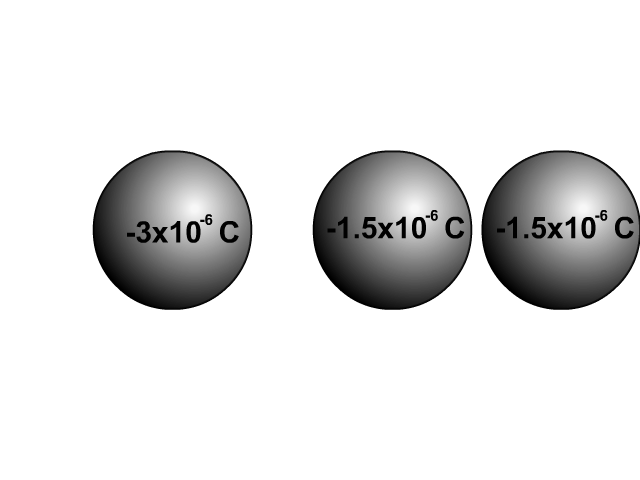
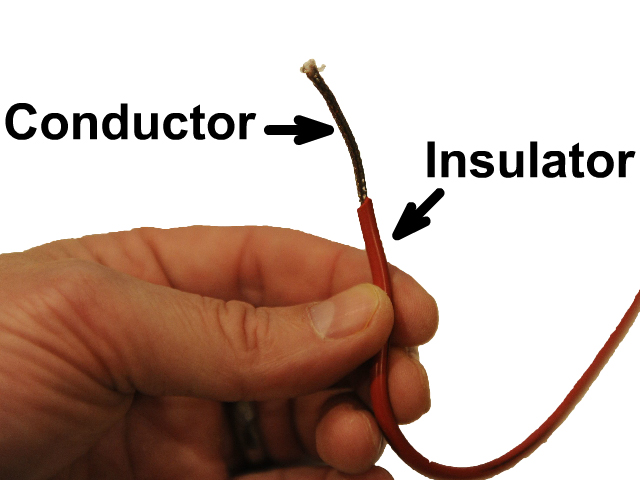
Electrical Insulators
Electrical Insulators are materials that do not let electrons flow freely. Insulators would be used for wire coatings or protective gloves for electricians. You can plug a device into the outlet without getting electrocuted because the wires are insulated as the one in this picture.
Common Electrical Insulators
- Rubber
- Plastic
- Glass
- Air
- Wood
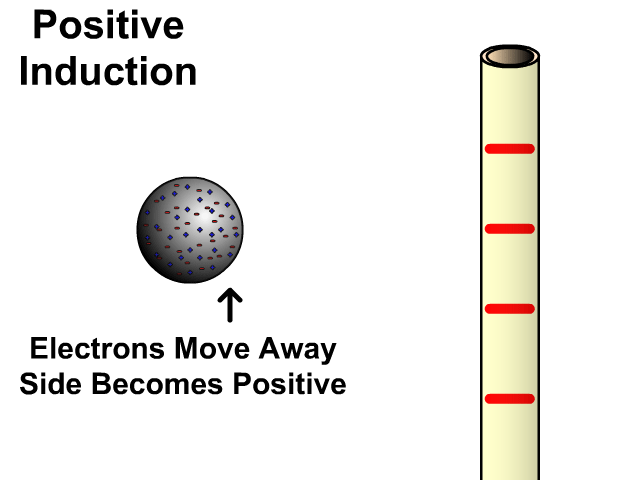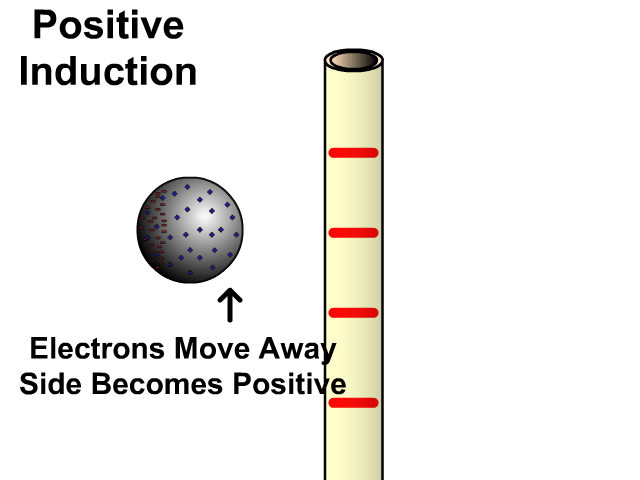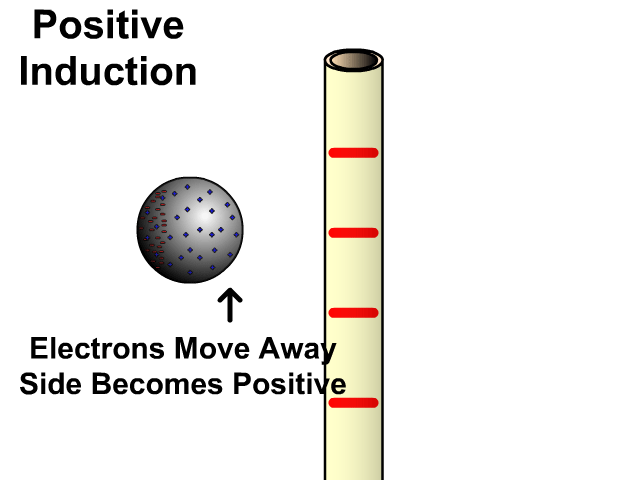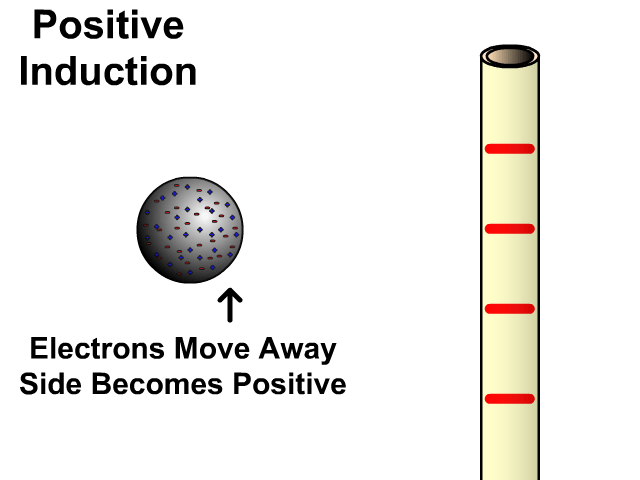
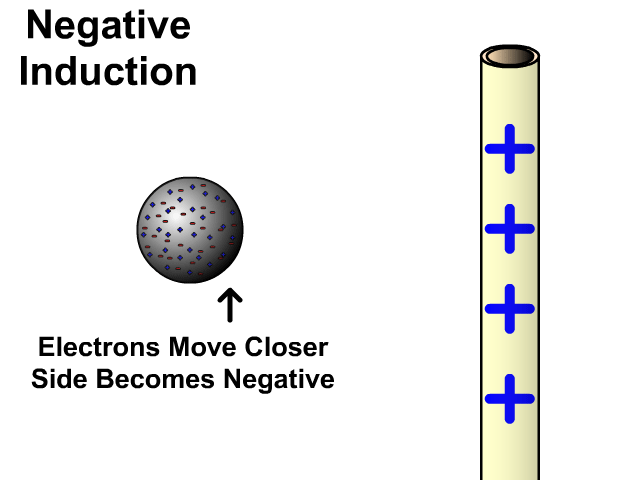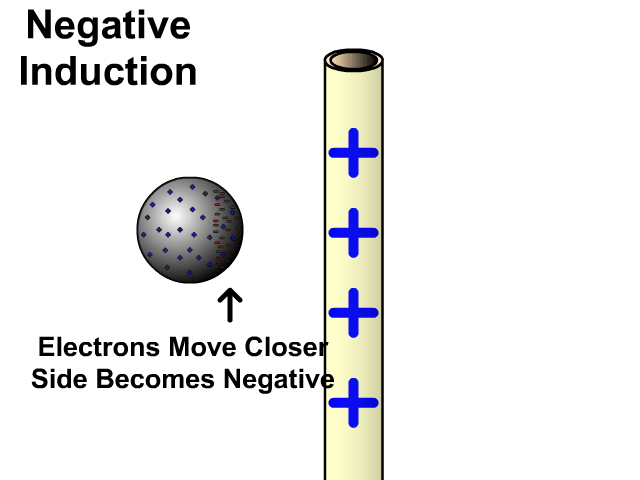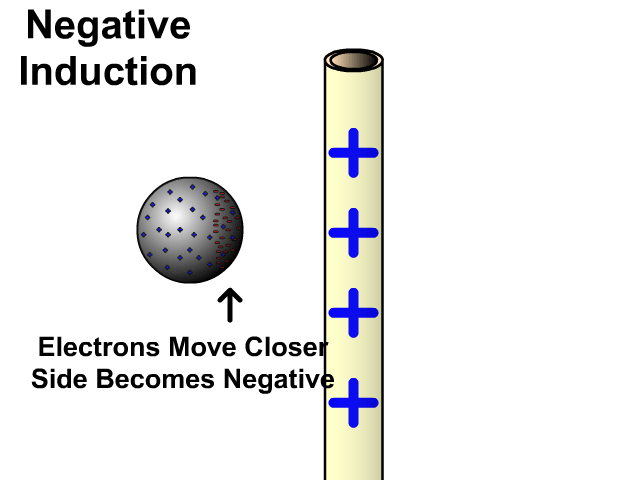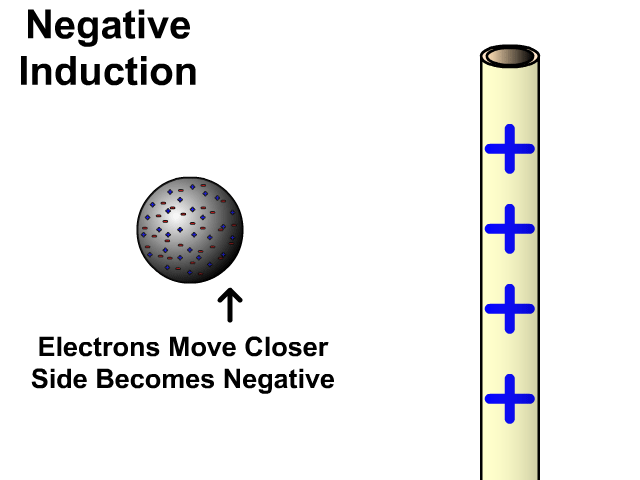
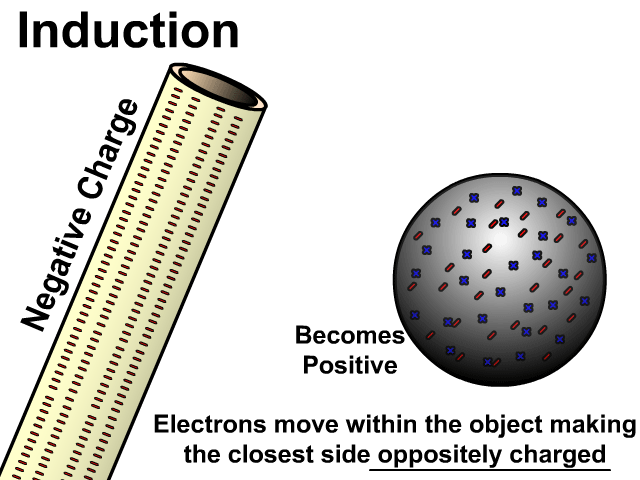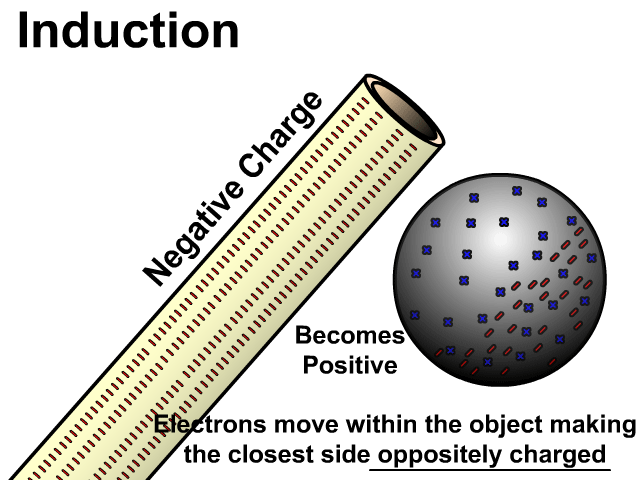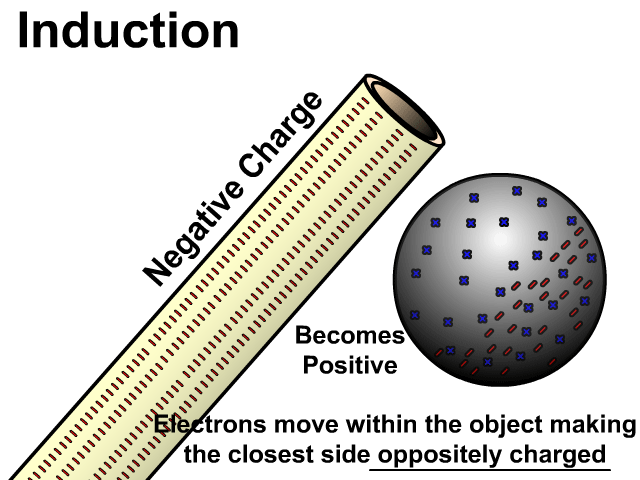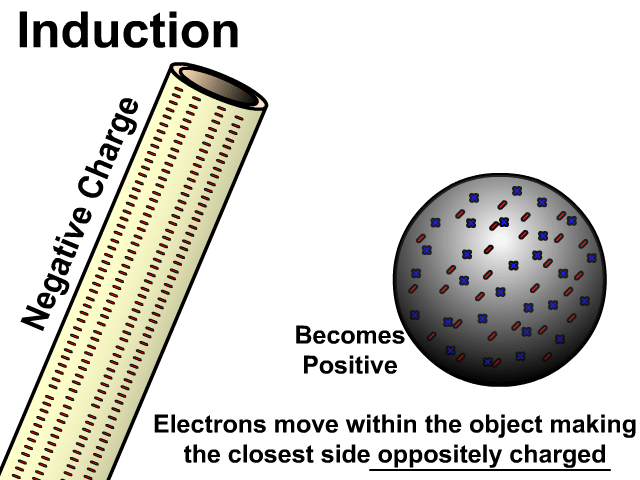
Charging By Induction
You can induce a charge in a neutral object by moving a charged object close to it. Induction creates a temporary and opposite charge in that other object with no contact. This is considered temporary because no electrons are transferred and neutrality returns when the close charged object is removed.
Charging By Induction Facts
- No contact
- Opposite charge
- Temporary (no electron transfer)
Charge When Charged By Induction
- Positive induces negative
- Negative induces positive
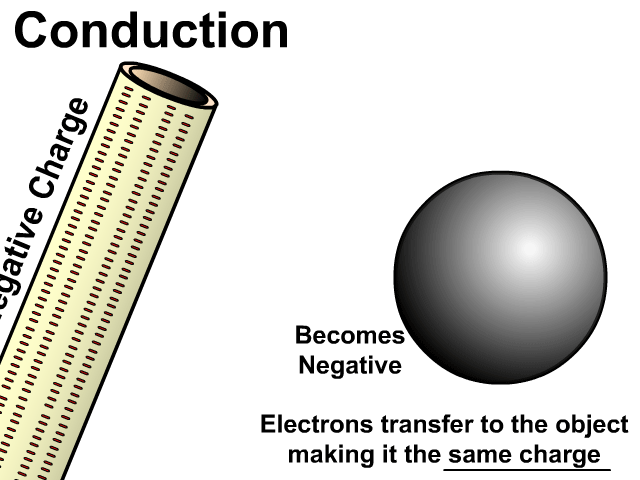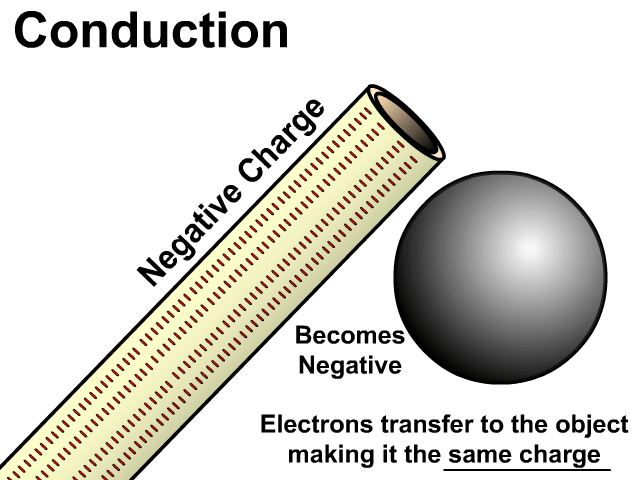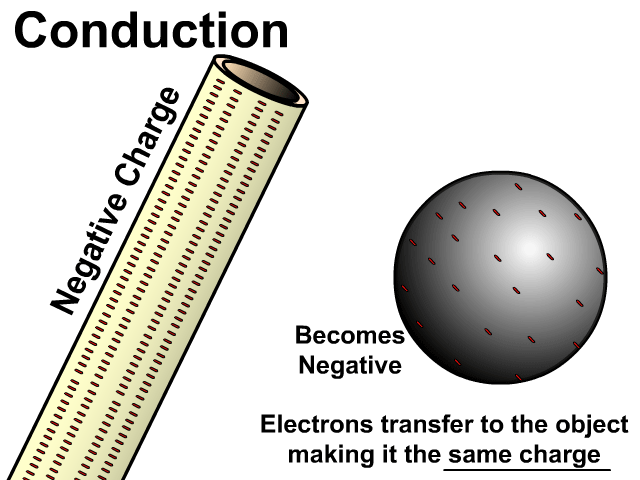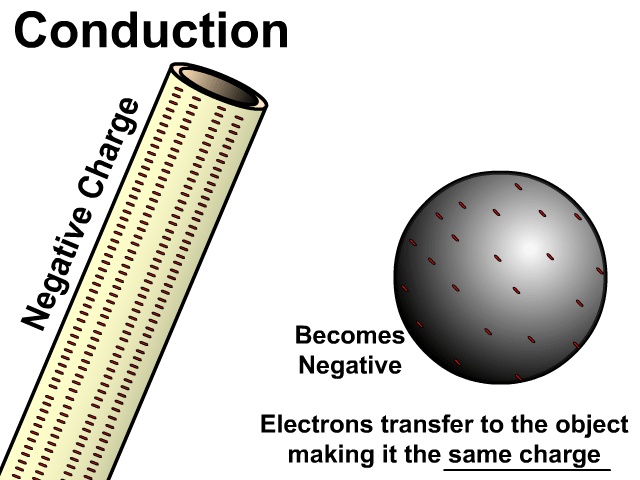
Charging By Conduction
Conduction occurs on a neutral object when a charged object is in contact with it.
During conduction the same charge is created in a neutral object. Electrons will transfer from a negative object to a neutral object making it negative. Electrons will be attracted by a positive object taking electrons from a neutral object making it positive.
Charging by conduction is considered permanent since electrons move to the new object until that object is grounded.
Charging By Conduction Facts
- Contact
- Same charge
- Permanent (with electron transfer)
Charge When Charged By Conducted
- Negative creates negative
- Positive creates positive
Models of Induction and Conduction Side By Side
In the model of charging by induction, protons are left in the model. Protons will always be there but models are meant to be simple. When electrons are repelled and move away while protons remain. This leaves the closest side positive. There is no contact, the negative induces a positive, and its temporary. It is temporary because electrons do not transfer to or from the object charged.
In the model of charging by conduction, protons were left off. Once again, protons are there but models are made to see just what is important. A negatively charged object has electrons that are transferred to the other object. This makes the other object have extra electrons and become negative as well. There is contact, the negative conducts a negative charge, and it is permanent. Permanent because electrons do transfer and stay on the newly charged object.

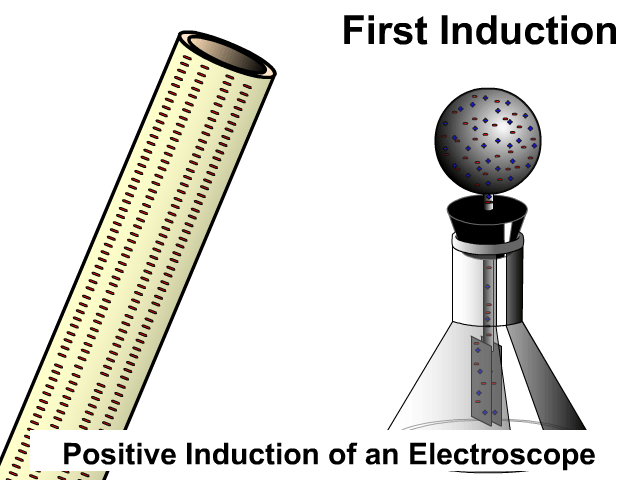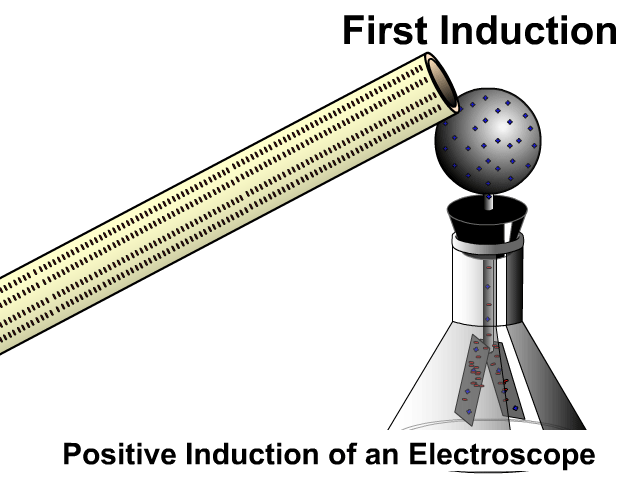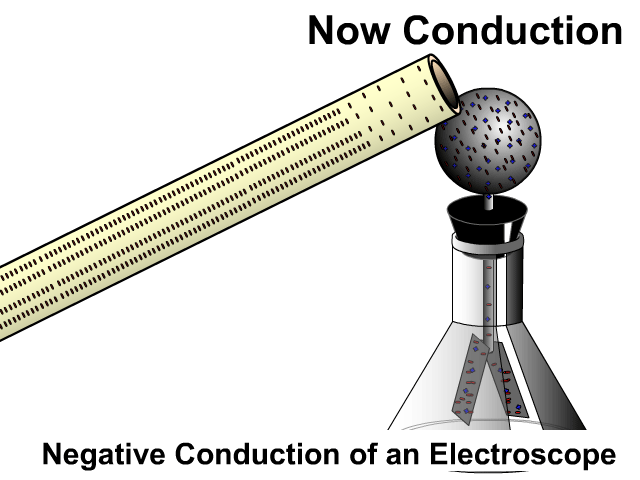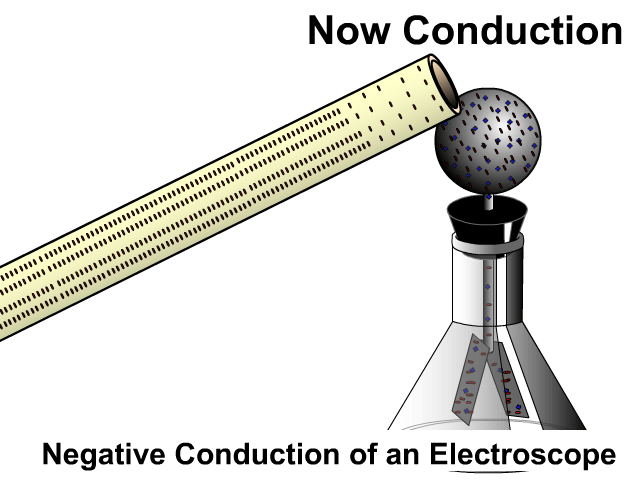
Induction then Conduction of an Electroscope
An electroscope is a composed of a conductive top sphere with a conductive path to the inside of an Erlenmeyer flask with two conductive light sheets of metal in close contact. When the leafs are charged they separate. The animation here starts with induction as the charged PVC pipe comes close and electrons on the electroscope sphere move away. As soon as contact occurs there is conduction, electrons leap off a charge object making it negative as well.
Grounding
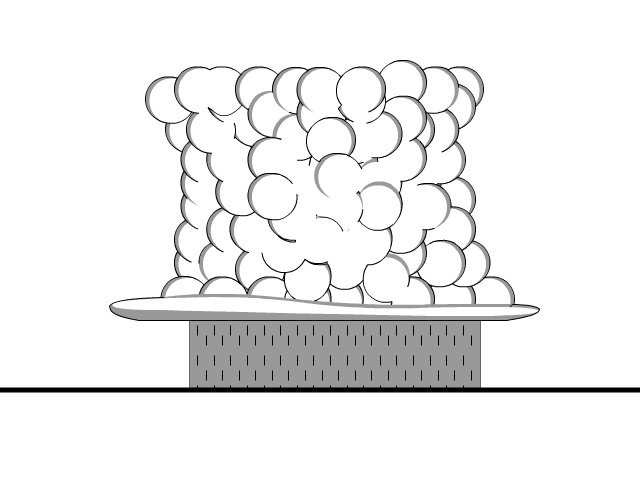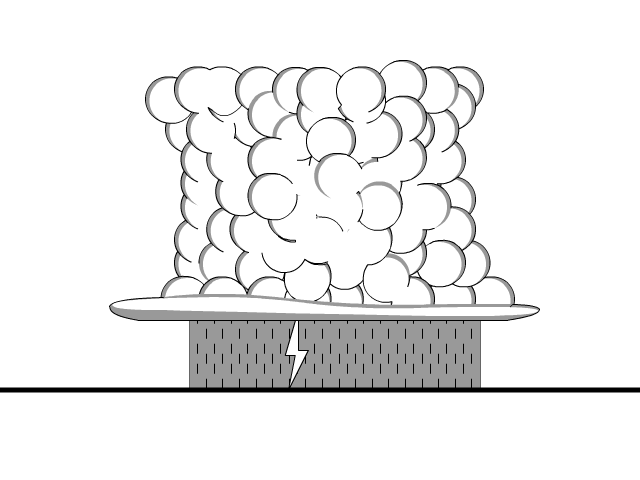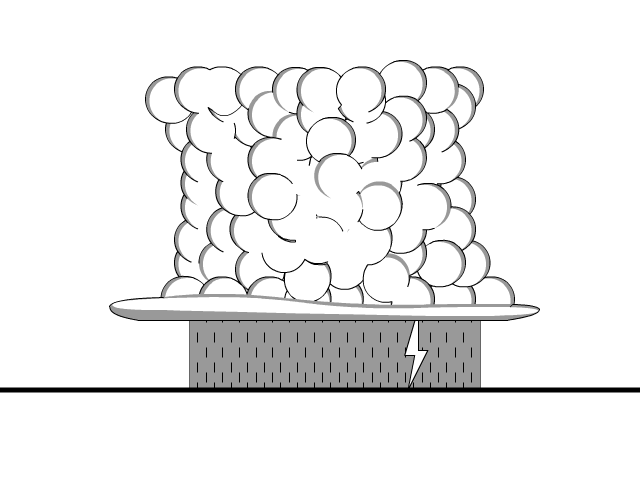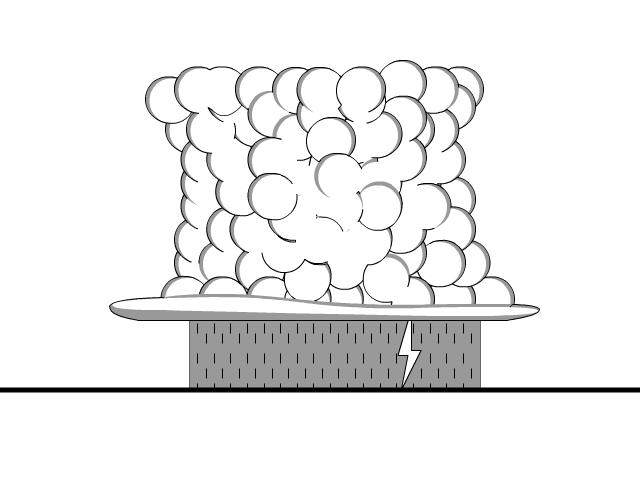
Grounding is the removal of a charge by producing a conductive path to the ground. The earth both accepts and gives electrons to neutralize objects.
- Electrons move from a negatively charged objects to the ground until the object is neutral
- Electrons move from ground to neutralize positively charged objects
Lightning is a form of grounding and on a lesser scale the same thing happens when you shock someone creating the arc (light of the lightning) and a bit of noise.
PhET Electrostatics Simulation
You can play around and observe charging by friction, charge interactions, and induction with the simulation below.
Find a basic lab sheet for the PhET Electrostatics Simulation below by clicking here
Electrostatics Quiz 1
Links
- On to Electrostatics Part 2 Coulomb's Law
- Back to the Electrostatics Main Page
- Back to the Stickman Physics Home Page
- Equation Sheet