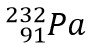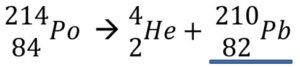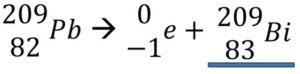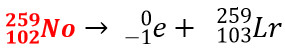Nuclear Decay
Learn about beta, alpha, and gamma decay. Go through practice examples following reactants and products and follow it up with half life practice.
Nuclear Decay Learning Targets:
- Describe the four fundamental forces in nature
- Explain reasons for nuclear decay
- Explain alpha, beta, and gamma radioactive decay comparatively using models
- Balance equations involving alpha, beta, and gamma radioactive decay
Four Fundamental Forces in Nature (Weakest to Strongest)
- Gravity: The weakest force in nature caused by the attraction of objects with mass. Gravity is only an attractive force which decreases the further objects are apart.
- Weak Force: A force that occurs at a very close distance within a nucleus and is responsible for beta decay.
- Electromagnetism: The second strongest force in nature caused by the attraction and repulsion of charged particles. This decreases the further the charges are apart and consists of attractions and repulsion.
- Strong Force: The strongest force in nature which works at a very close distance holding the protons and neutrons of the nucleus together.
You can read more about fundamental forces following this link.
Reasons for Decay
In the nucleus the electrostatic force or two positive protons so close together would cause the nucleus to violently separate if it was not for the stronger strong force which is stronger acting like a cement holding the nucleons together.
Reasons for Radioactive Decay
- Large nuclei (Z > 82) : electrical forces of repulsion are greater than strong forces of attraction if a nucleus is too massive beyond 82 protons with associated neutrons.
- Wrong neutron to proton ratio: more neutrons are necessary to help stabilize an atom as it gets bigger and there are more protons interacting.
A Radioactive Isotope
- Has an unstable nucleus
- Spontaneously emits a particle and decays into another element (to become more stable)
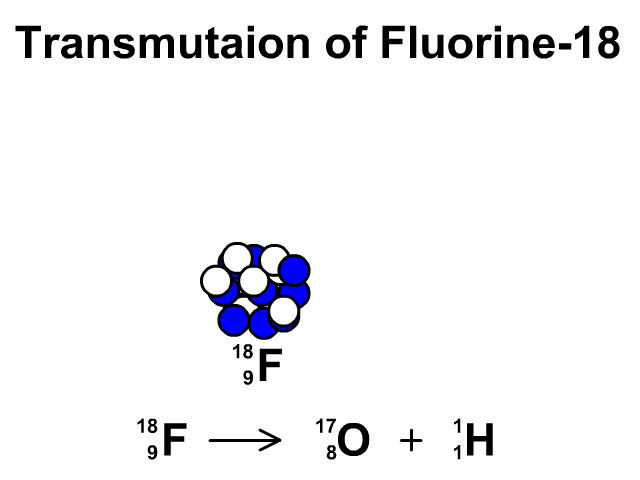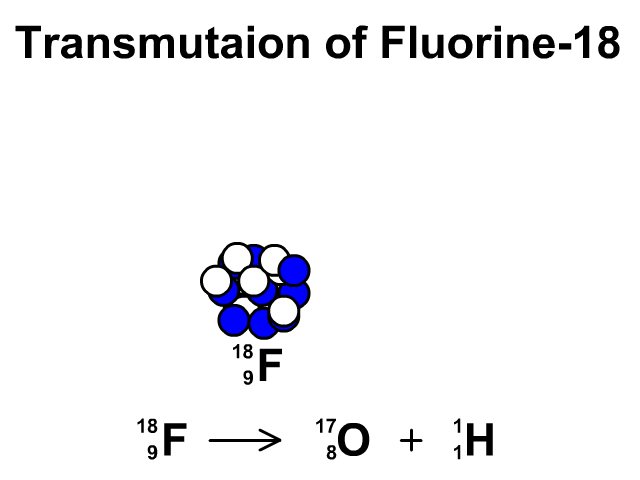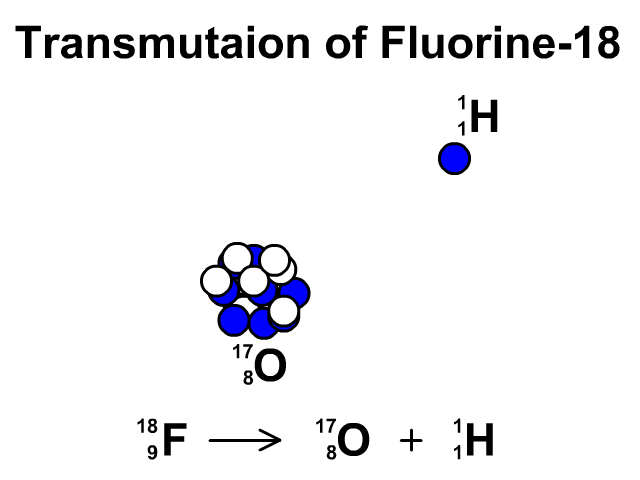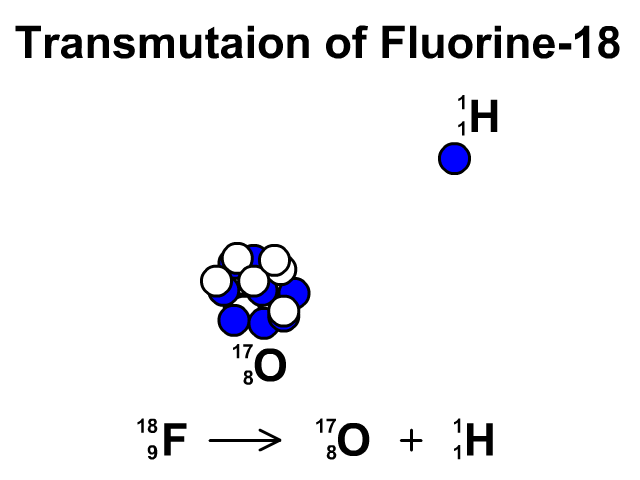
Transmutation
A transmutation is the change of one element into another through radioactive decay.
In this animation you see Fluorine-19 release a proton and transmutate into Oxygen-18.
Three Types of Radioactive Decay
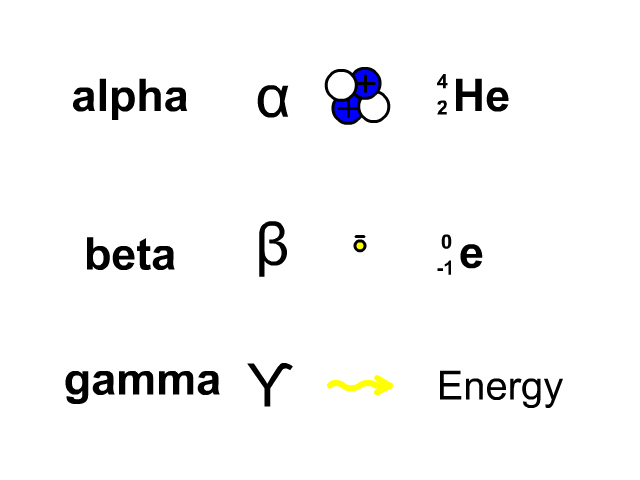
Three types of decay are alpha, beta, and gamma decay
- Alpha decay: release of a helium nucleus with two protons and two neutrons. The decaying element loses two protons from its atomic number and four nucleons from its atomic mass.
- Beta decay: release of an electron, called a beta particle, from a neutron which results in a neutron becoming a proton. The decaying element gains a proton in its atomic number and the atomic mass stays the same.
- Gamma decay: release of energy as an atom becomes more stable. The decaying element stays the same as it just loses energy in the form of a gamma ray.
Look at the picture for the symbols or molecular representation you will see in the following example decay reactions.
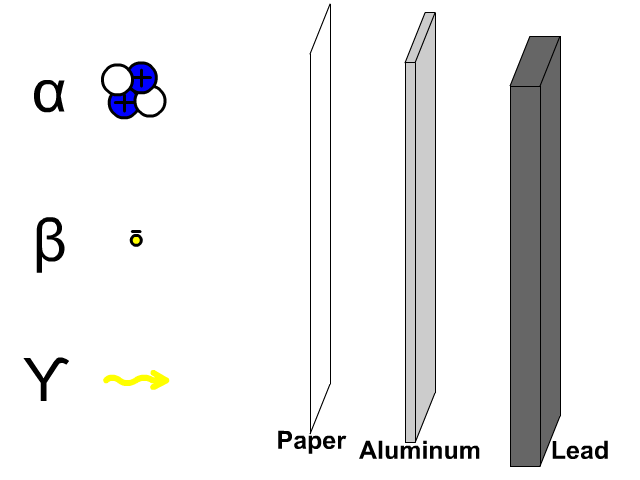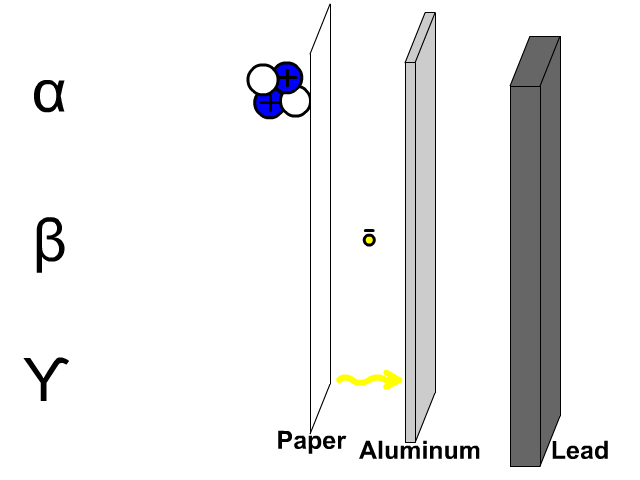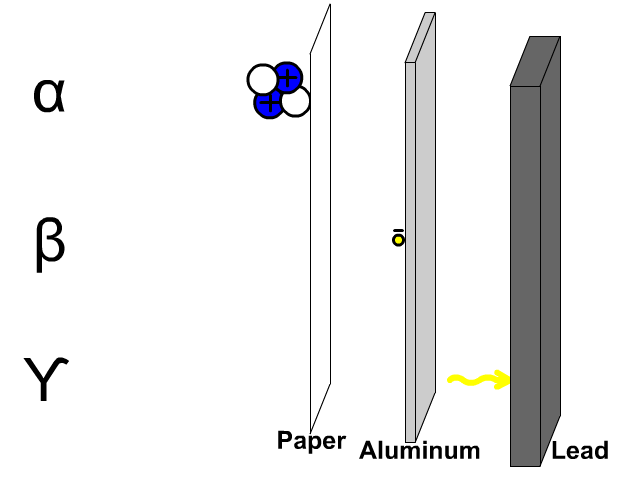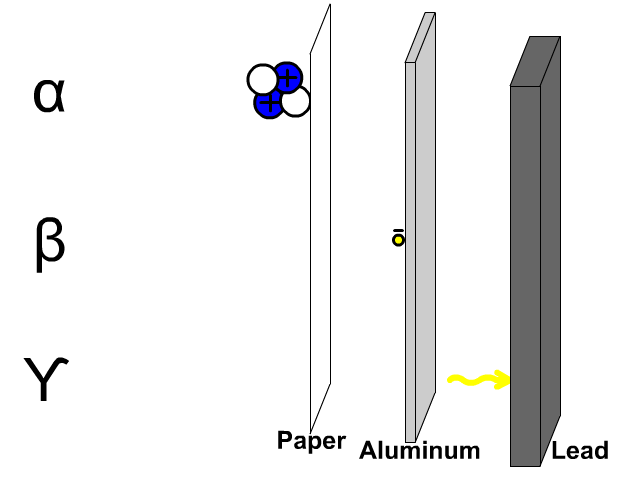
Penetration of Alpha, Beta, and Gamma Particles
- Alpha particles, composed of two protons and two electrons, are comparatively large so a thin paper barrier will stop alpha particles.
- Beta particles, composed of electrons, can get through paper but have trouble penetrating a thicker sheet of aluminum.
- Gamma particles, composed of energy, have trouble getting through thick dense lead.
A thick sheet of lead is often used to protect humans from all form of radiation since it is a large dense molecule and hard for most forms of radioactive emissions to penetrate through.
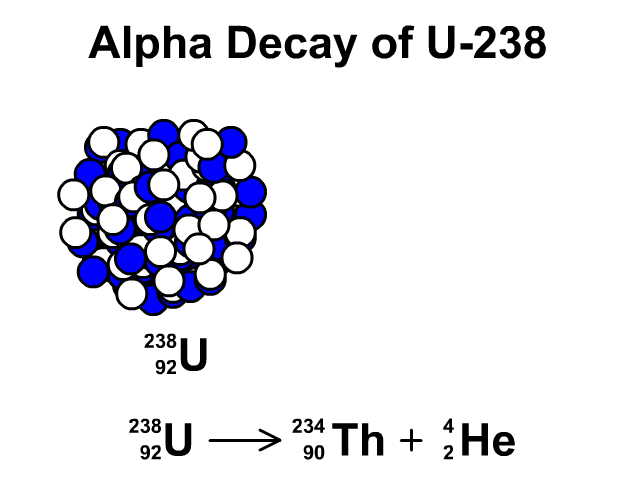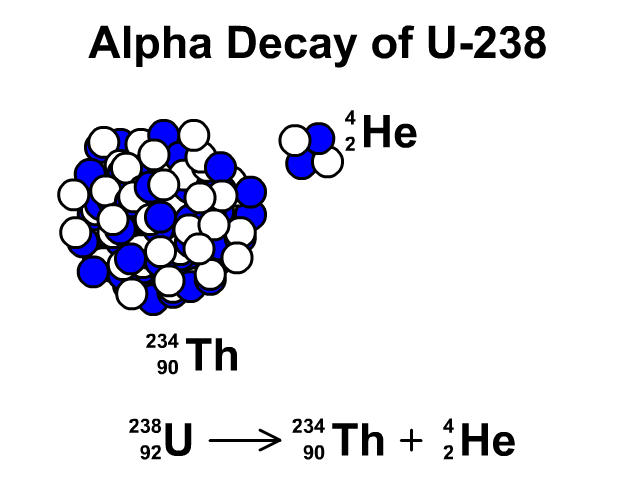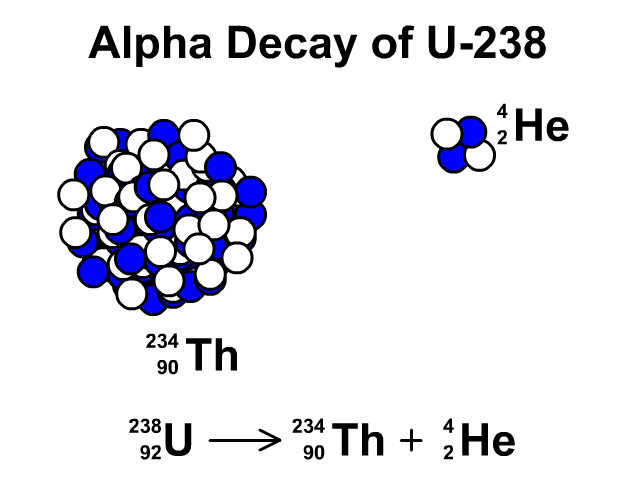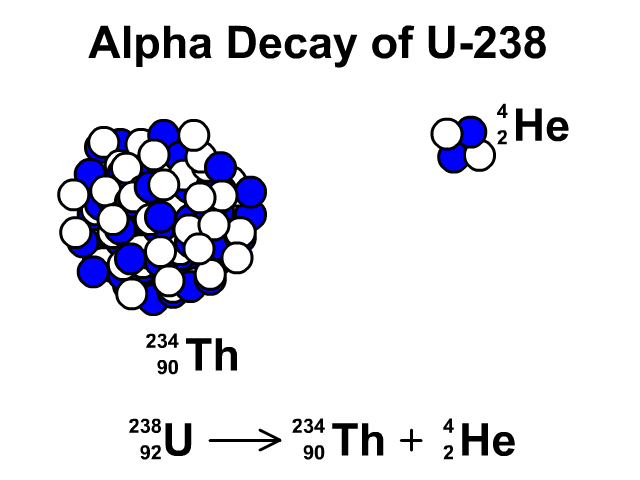
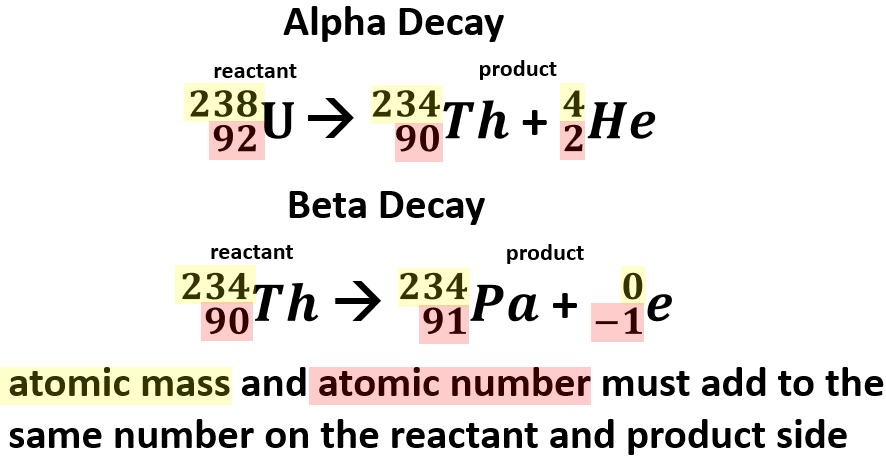
Alpha Decay of Uranium-238
- Uranium-238 has 92 protons and 146 neutrons with a total atomic mass of 238.
- As Uranium-238 loses an alpha particle it loses two protons and two neutrons. The atomic number goes down by two for the loss of two protons. The atomic mass goes down by four from releasing four nucleons (two protons and two neutrons). The resulting element is Thorium-234.
- Thorium-234 has 90 protons and 144 neutrons with a total atomic mass of 234
Observe the alpha decay and representative equation in our animation.
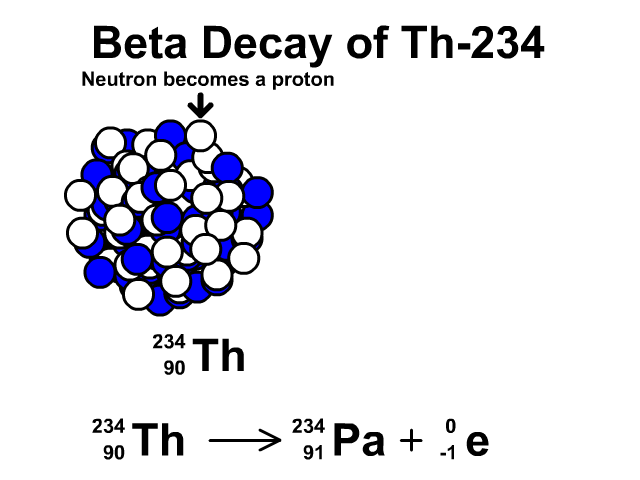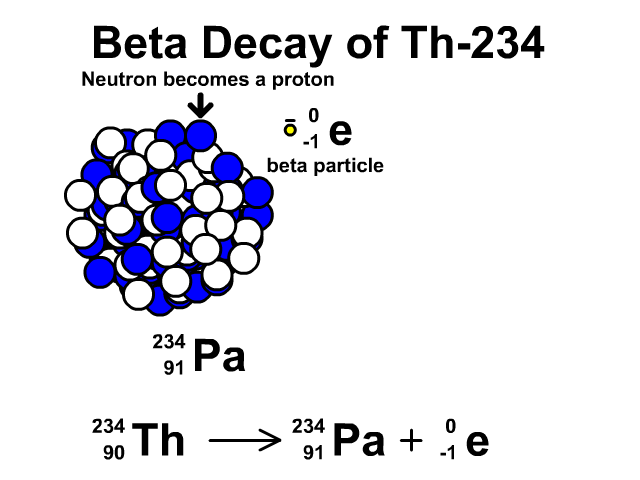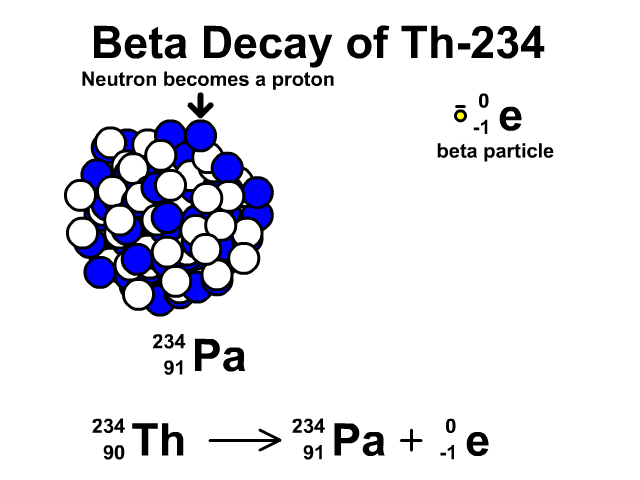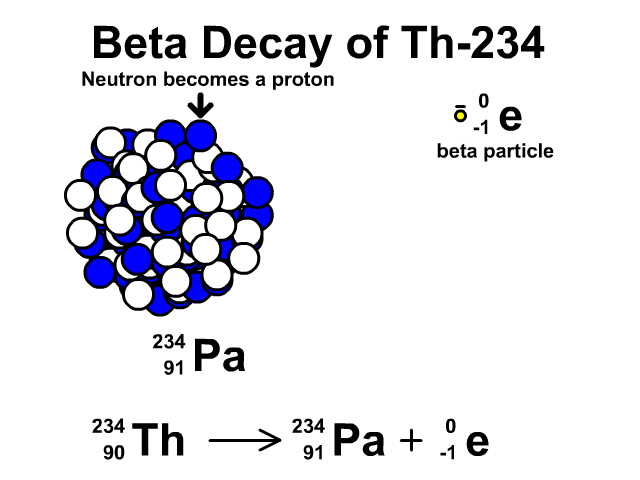
Beta Decay of Thorium-234
- Thorium-234 has 90 protons and 144 neutrons with a total atomic mass of 234
- As Thorium-234 undergoes beta decay, a neutron becomes a proton. The atomic number goes up one as the new element has an additional proton. The atomic mass stays the same as the new element has the same number of nucleons. One extra proton and one less neutron, which have relatively the same mass. An electron is very small compared to a nucleon. The resulting element is Protactinium-234.
- Protactinium-234 has 91 protons and 144 neutrons with a total atomic mass of 234
Observe the beta decay and representative equation in our animation.
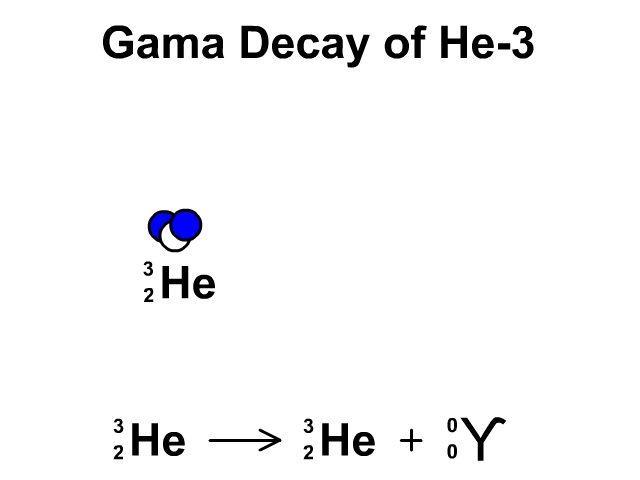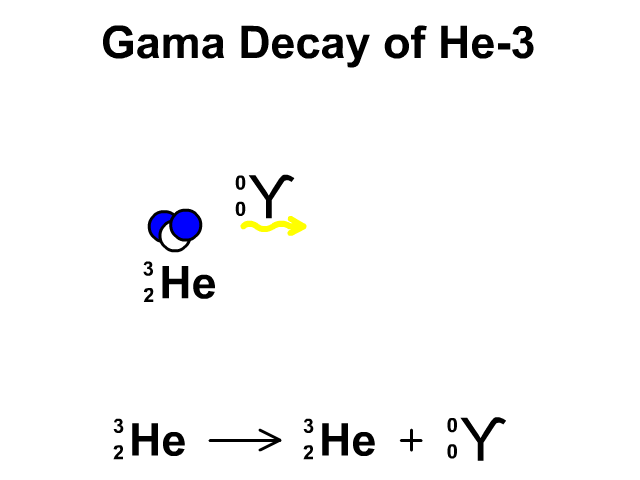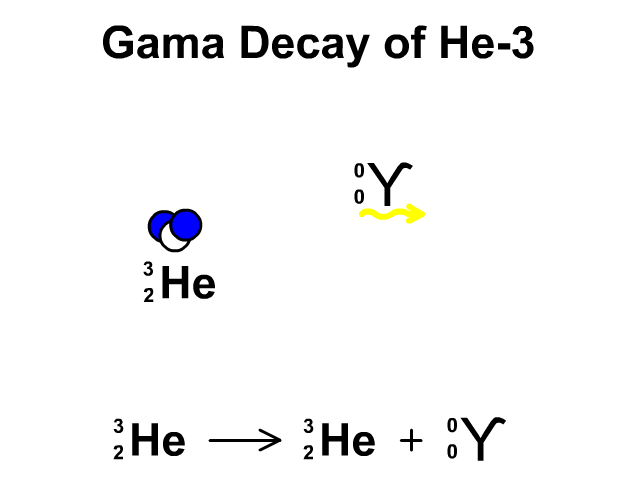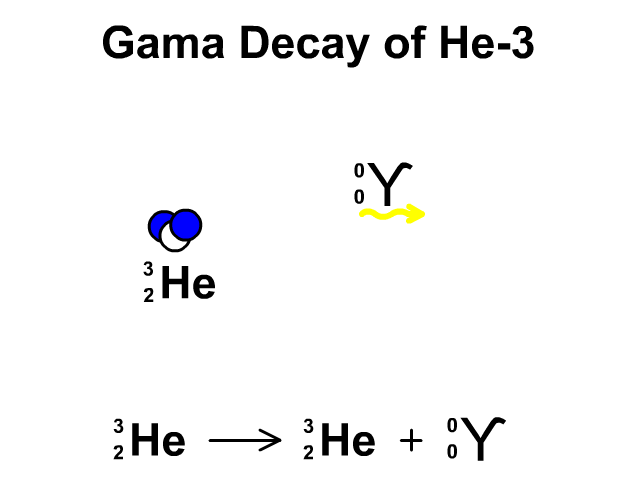
Gamma Decay of Helium-3
- Helium-3 has 2 protons and 1 neutron with a total atomic mass of 3
- Helium-3 releases a gamma ray, releasing energy, as it undergoes gamma decay. The resulting element is remains Helium-3.
Observe the gamma decay and representative equation in our animation.
Balanced Equation
Equations must be balanced. In nuclear decay reactions you must be certain that all the combined atomic mass of the reactants equals the combined atomic mass of the products. You must also be certain that the combined atomic number of the reactants equals the combined atomic mass of the products.
In alpha decay of U-238
- Atomic Mass: 238 = 234 + 4
- Atomic Number: 92 = 90 + 2
In beta decay of Th-234
- Atomic Mass: 234 = 234 + 0
- Atomic Number: 90 = 91 + (-1)
Notice for beta decay you must add a proton to compensate for the electron lost in the math.
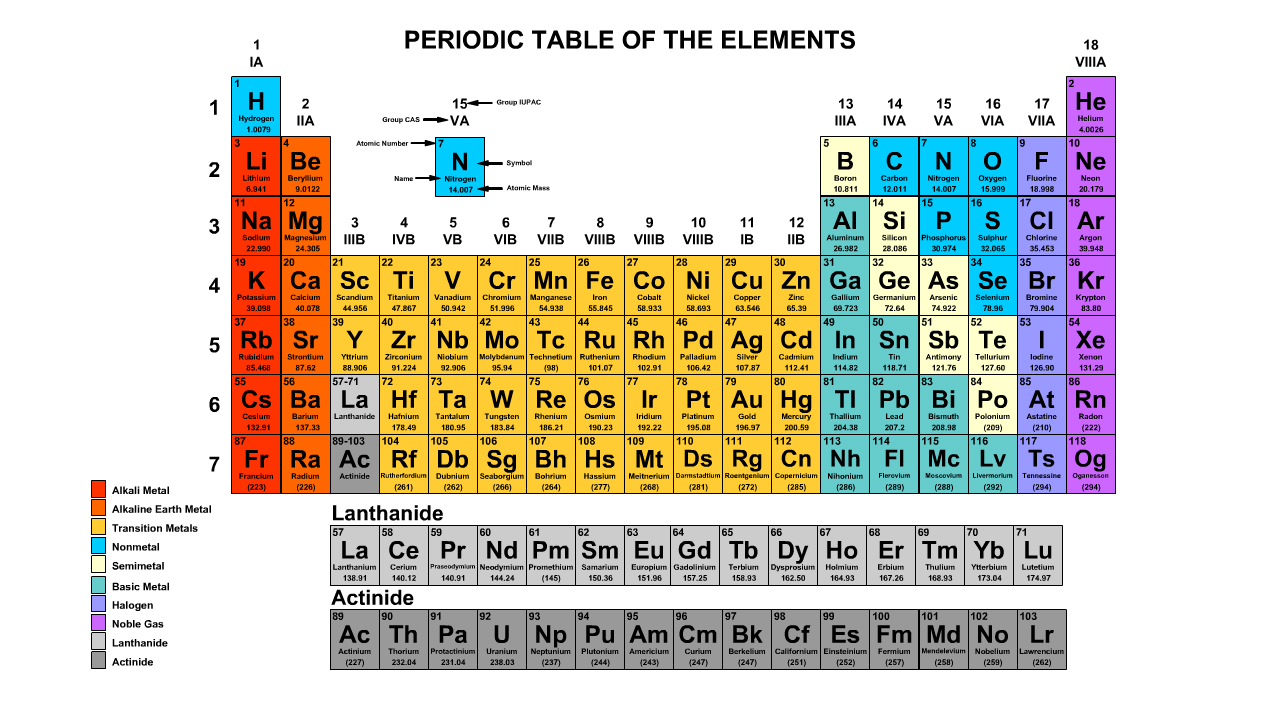
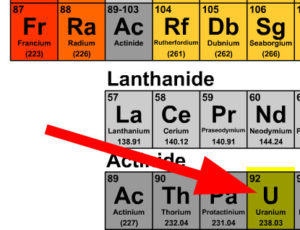
Use the periodic table below or print one here for reference on the guided examples and problem set
Balancing Nuclear Decay Examples
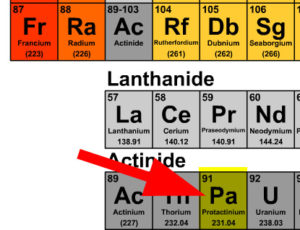
Guided Example A: Find the missing product in Thorium-232 beta decay
- Balance the atomic mass 232 = _____ + 0, the unknowns atomic mass is 232 because 232 = 232 + 0
- Balance the atomic number 90 = _____ + -1, the unknowns atomic number is 91 because 90 = 91 + (-1)
- Find the element that has the atomic number 91 on the periodic table which is Pa

- Using the atomic mass solved for in step one and not the average from the periodic table, the missing product is

Guided Example B: Find the reactant that decays to the products Thorium-234 and Helium-4
- Balance the atomic mass _____ = 234 + 4, the unknowns atomic mass is 238 because 238 = 234 + 4
- Balance the atomic number _____ = 90 + 2, the unknowns atomic number is 92 because 92 = 90 + 2
- Find the element that has the atomic number 92 on the periodic table which is U

- Using the atomic mass solved for in step one and not the average from the periodic table, the missing product is

Decay Reactions Problem Set
1. Balance the alpha decay of Po-214 reaction below
2. Balance the beta decay of Pb-209 reaction below
3. Balance the alpha decay of Americium-243 reaction below
4. Balance the alpha decay of Promethium-144 reaction below
5. Balance the beta decay of Einsteinium-252 reaction below
6. Balance the nuclear equation when a parent particle beta decays to form Neptunium-238
7. Balance the nuclear equation when a parent particle beta decays to form Lawrencium-259
8. Balance the nuclear equation when a parent particle beta decays to form Plutonium-244
9. Balance the nuclear equation when a parent particle alpha decays to form Seaborgium-261
Links
- On to Half-Life
- Back to the Main Nuclear Physics Page
- Back to the Stickman Physics Home Page
- Check out our StickMan Physics YouTube Channel
- Print a Periodic Table
- Equation Sheet