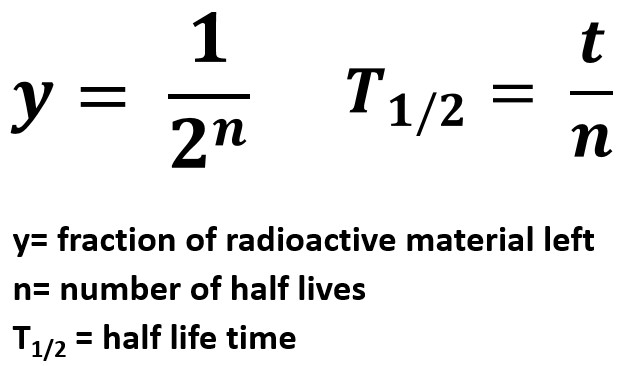Half-Life
Radioactive elements goes through decay at different rates. The time it takes for the element to degrade by half is called a half-life. Learn more here.
Half-Life Learning Targets:
- I understand the meaning of a radioactive isotope half-life
- I can determine the amount of radioactive substance remaining after an amount of time (mathematically and visually with a table)
- I can determine the amount of radioactive substance remaining after a number of half lives (mathematically and visually with a table)
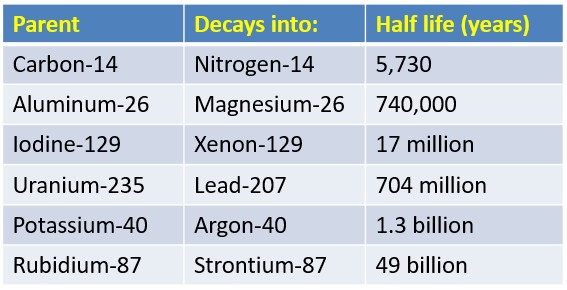
Half Life of Common Radioactive Isotopes
A half-life is the time it takes for half of the radioactive sample to decay.
- Half lives range from a fraction of a second to billions of years.
- The length of a half life is shorter the less stable the radioactive isotope.
Radiometric Dating
Radiometric dating is a process of determining the age of an object by the amount of radioactive isotope that has degraded from an initial sample.
Carbon-14 is a radioactive form of carbon used for radiometric dating of once living organisms.
Carbon-14 has a half-life of 5,730 years. All life molecules have Carbon, testing the amount of radioactive carbon present in a sample can determine its age.
An archaeologist that tests a bone and finds it has half the normal amount of radioactive carbon-14 just determined its age is 5,730 years old.
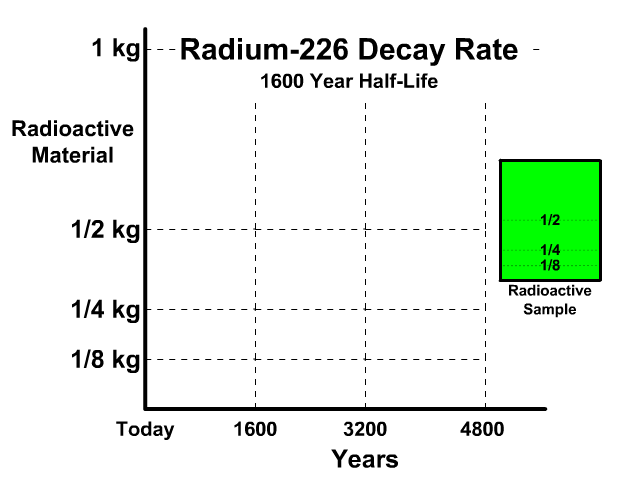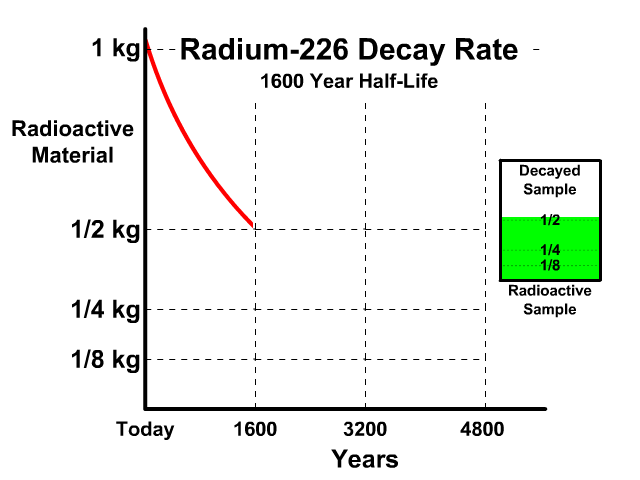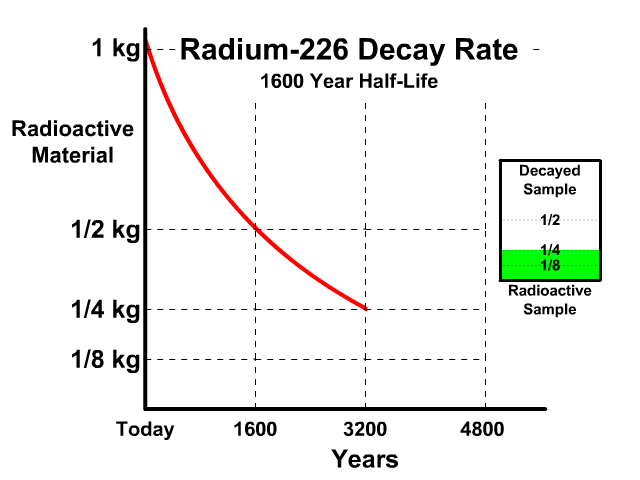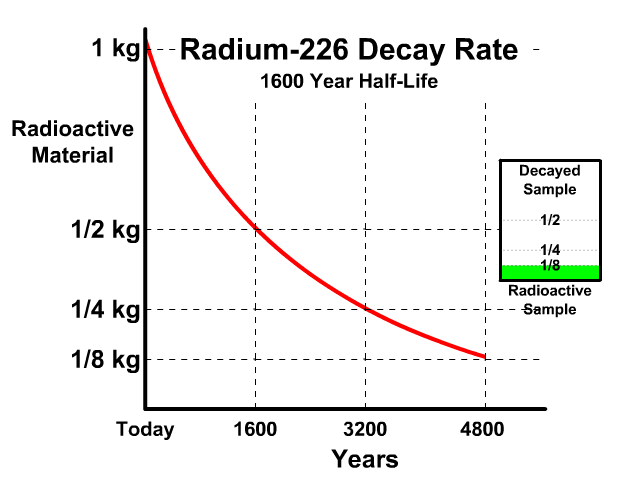
The half-life of Radium-226 is 1600 years. This means a 1 kg radioactive sample today would decay to a 1/2 kg radioactive sample after 1600 years.
Analyze the table along with the animation to identify the pattern and relationship between number of half-lives, kilograms of radioactive sample size, nonradioactive sample size, and percent decayed.
| Half Lives | Radioactive | Nonradioactive | Percent Decayed |
| 0 | 1 kg | 0 kg | 0% |
| 1 | 1/2 kg | 1/2 kg | 50% |
| 2 | 1/4 kg | 3/4 kg | 75% |
| 3 | 1/8 kg | 7/8 kg | 87.5% |
Half Life Equations
We will work through a few sample problems on this page using a table form and the following equations.
y=1/(2n)
T1/2 = t/n
- y represents the fraction of radioactive material remaining
- n represents the number of half lives
- T1/2 represents the length of one half life
Guided Half-Life Example
A. How much of the original radioactive material remains after four half-lives?
Givens List:
-
- n = 4
- y=?
Equation:
-
- y=1/(2n)
Work:
-
- y=1/(24)
- y= 1/16 or 0.0625
B. How much of a 56 gram radioactive sample radioactive remains after four half-lives?
First: Find the fraction left
Givens List:
-
- n = 4
- y=?
Equation:
-
- y=1/(2n)
Work:
-
- y=1/(24)
- y=0.0625
Second: Multiply the fraction by the original sample size
0.0625 x 56 grams = 3.5 grams of a radioactive sample remains
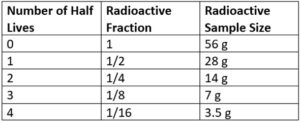
Using a table to answer example A and B above
#1 Make a table as seen below starting with 0 half lives and columns for any necessary information. Fill in one in this column if asked about a fraction, fill in 100% if asked about a percent.
#2 Fill in the table with an extra row for every half life until you get to the number of half-lives in the question
#3 The completed table here could be used to answer questions about the fraction or sample remaining for up to four half-lives of a 56 gram sample.
C. How long is a half-life for a substance that has 2.5 half lives every 50,000 years?
Givens:
-
- n = 2.5
- t = 50,000 years
- T1/2 = ?
Equation:
-
- T1/2 = t/n
Work:
-
- T1/2 = 50000/2.5
- T1/2 = 20,000 years
D. A 100 gram unknown radioactive material has a half-life of 4000 years. How many grams of the radioactive sample will remain after 20,000 years?
Step 1: Determine the number of half-lives
Givens:
-
- T1/2 = 4000 years
- t = 20,000 years
Starting Equation:
-
- T1/2 = t/n rearranges to n = t/T1/2
Work:
-
- n = 20000/4000
- n = 5
Step 2: find the fraction left after five half-lives
Givens:
-
- y = ?
- n = 5
Equation:
-
- y =1/(2n)
Work:
-
- y =1/(25)
- y = 0.03125
Step 3: Multiply the fraction by the original sample mass
0.03125 x 100 g = 3.125 grams
Example Problems
Note: You can use a table or math for any of the following examples
1. What percentage of radioactive substance remains after ten half-lives?
2. If a substance starts out with 400g, how much remains after five half-lives?
3. How many complete half-lives have occurred if 12.5% of the original radioactive material remains?
4. How many complete half-lives have occurred is 18.75 grams of an originally 600 gram radioactive substance remains?
Links
- On to Fission and Fusion
- Back to the Main Nuclear Physics Page
- Back to the Stickman Physics Home Page
- Check out our StickMan Physics YouTube Channel
- Equation Sheet