Fission and Fusion
Fission releases energy produced in a nuclear reactor. Fusion is the energy of the stars and the reason our sun burns bright. Learn more here.
Fission and Fusion Learning Targets
- Understand the difference between fission and fusion using a model
- Be able to recognize fission, fusion, alpha decay, beta decay, or gamma decay in an example
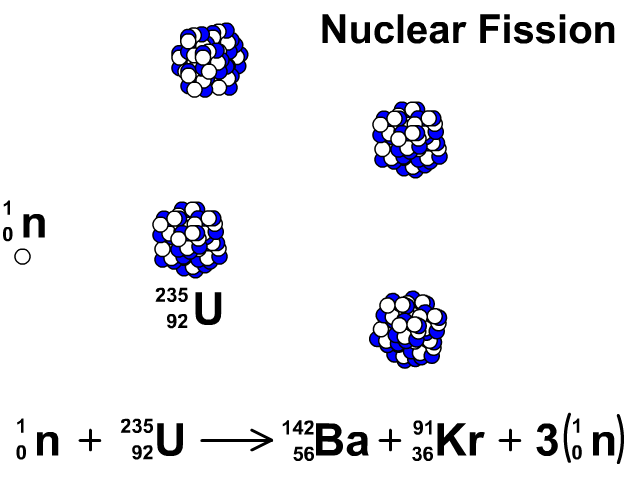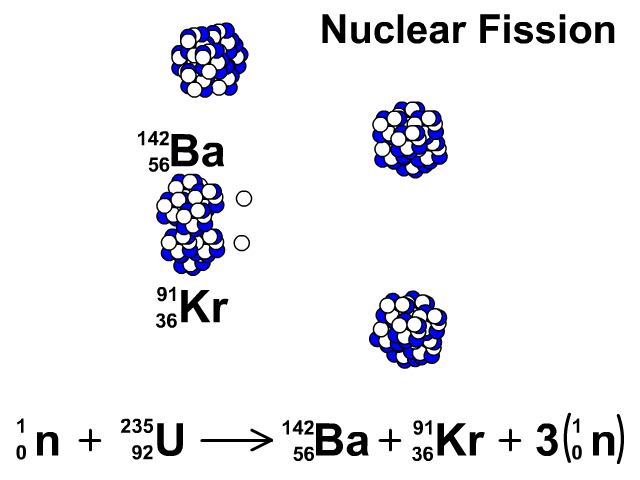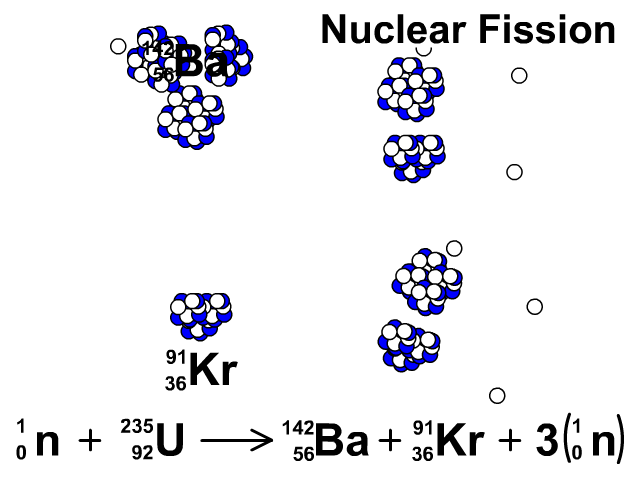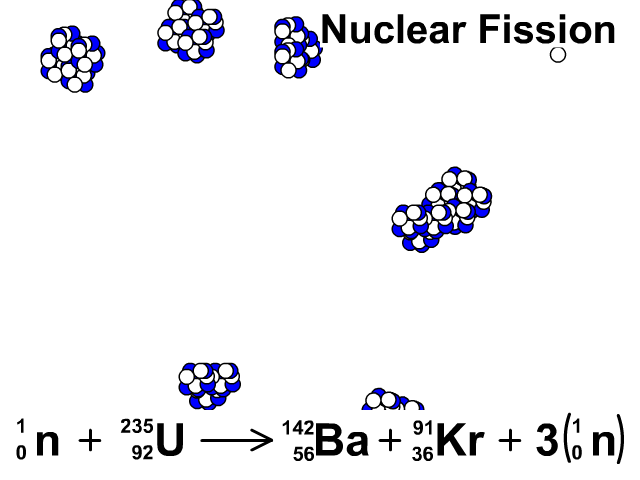
Nuclear Fission
During nuclear fission, neutrons bombard heavy nuclei splitting them forming two smaller atoms.
The mass of product particles is slightly less than the mass of the reactant. This mass is converted into energy which can be calculated using Albert Einstein’s famous equation E=mc2. Energy released equals the mass lost times the speed of light squared.
In our animation, neutrons bombard heavy U-235 splitting into Ba-142, Kr-91, and three neutrons. The neutrons released continue to create fission reactions with U-235.
A spontaneous reaction that will continue as long as there is a critical mass of U-235 to split.
Critical mass is minimum mass of fissionable material required for a chain reaction. The fissionable material in our animation is Uranium-235.
Fission reactions are done in in a reactor to contain the extreme amounts of energy produced. A nuclear bomb is the result of this reaction in an unrestrained environment.
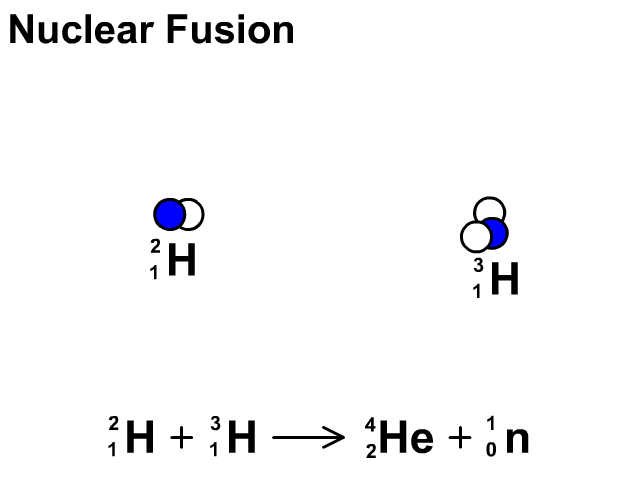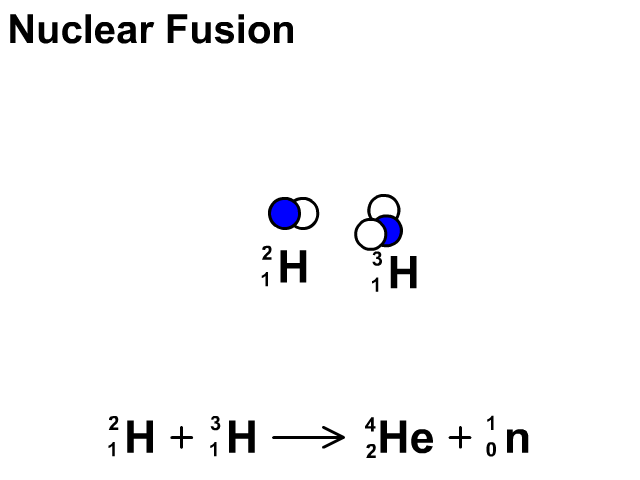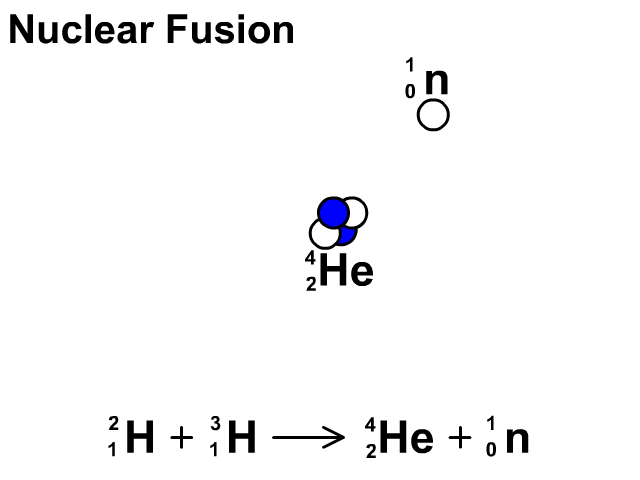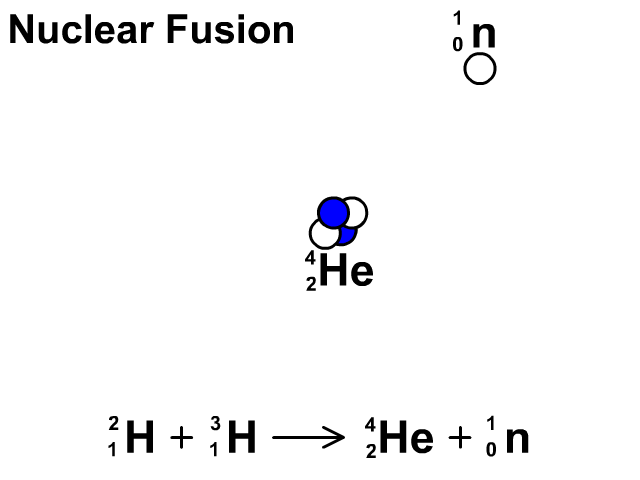
Nuclear Fusion
Nuclear fusion is the combination of small nuclei into larger nuclei which releases large amounts of energy.
- Nuclear fusion can release up to 10 times more energy than nuclear fission. Nuclear fusion occurs naturally and is the source of energy in our sun and other stars.
- In the fusion reaction, two smaller Hydrogen atoms are fused together to form a larger Helium and a neutron is released.
- Nuclear fusion requires high temperatures, which is readily available in a star. We currently have not discovered a way to maintain temperatures required to sustain nuclear fusion without vaporizing the container of the fusion reaction. Today many are scientists are researching ways to create nuclear fusion under sustainable conditions.
- As of 2020, research is ongoing at companies like Lockheed Martin to create unlimited energy through fusion. Current nuclear technology only allows the use nuclear fission as an energy source.
Practice Questions
Answer the following nuclear decay or reaction questions related to alpha decay, beta decay, gamma decay, fission, or fusion.
- Reference our Nuclear Decay page for more support on alpha, beta, and gamma radiation by clicking here
1. Which nuclear decay or reaction does the following formula represent?
2. Which nuclear decay or reaction does the following formula represent?
3. Which nuclear decay or reaction does the following formula represent?
4. Which nuclear decay or reaction does the following formula represent?
5. Which nuclear decay or reaction does the following formula represent?
6. Which nuclear decay or reaction does the following formula represent?
7. What type of reaction creates the energy of stars?
8. What type of reaction produces energy of nuclear reactors in the year 2020?
9. What type of reaction releases an electron as a neutron becomes a proton?
10. What type of radioactive decay releases energy only?
11. Which reaction would produce more energy per reaction, fission or fusion?
Links
- Back to the Main Nuclear Physics Page
- Back to the Stickman Physics Home Page
- Check out our StickMan Physics YouTube Channel
- Equation Sheet