Atom and Periodic Table
Facts about atoms can be determined from the periodic table looking location, the atomic number, and atomic mass.
Atom and Periodic Table Learning Targets
- Know the basic subatomic components of an atom
- Be able to read a periodic table to determine element composition
- Understand the difference between atomic number and atomic weight
- Calculate the composition of an isotope using nucleons in the name and the periodic table
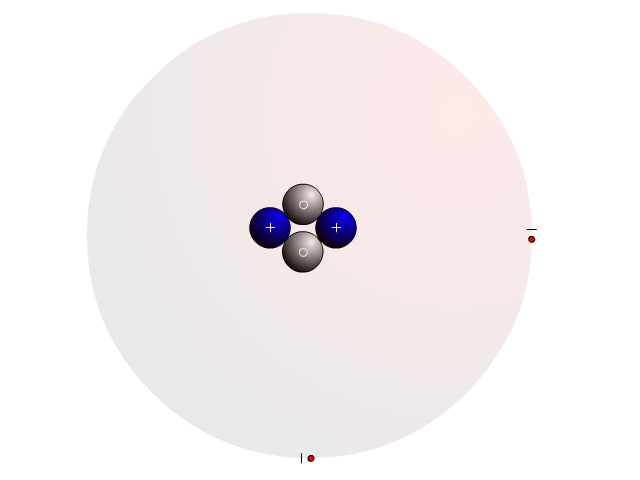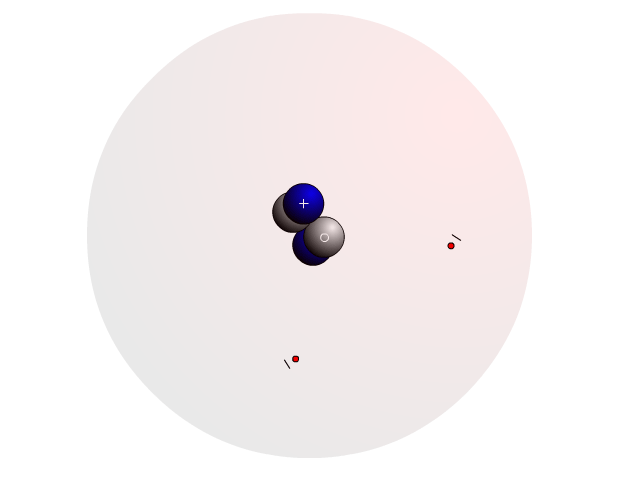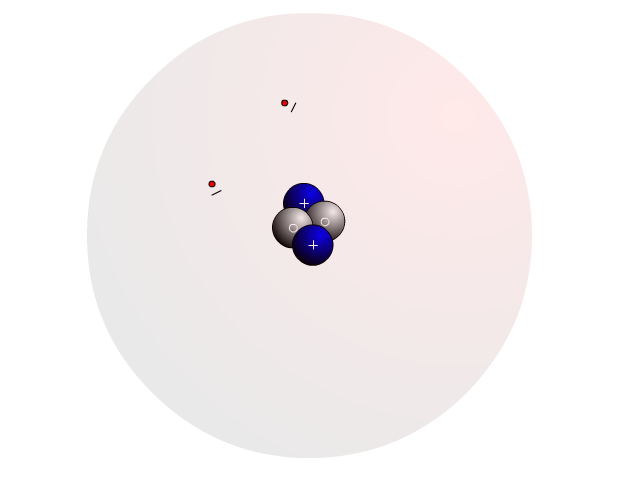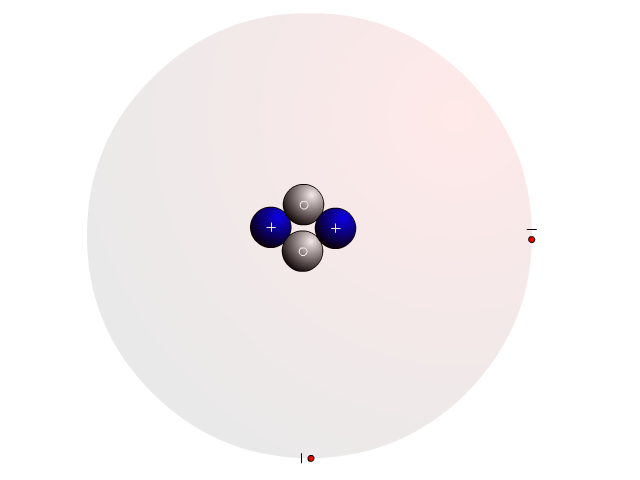
The Atom
In an atom, you find a nucleus with protons and neutrons. Outside the nucleus, you find regions that electrons will most likely occupy called orbitals.
The following table provides information about subatomic particles
| Masses and Charges of Subatomic Particles | ||
| Mass | Charge | |
| Proton | 1.673x10-27 kg | +1.602 x 10-19 C |
| Neutron | 1.675x10-27 kg | 0 C |
| Electron | 9.109x10-31 kg | -1.602 x 10-19 C |
Atom Components
In the nucleus you find protons with a positive charge and neutrons that are neutral (no charge). Strong nuclear forces hold the nucleus together.
Elements undergo changes to reach a higher level of stability:
- Bonding: Electrons can be shared with other atoms
- Radioactive Decay: Release in energy or change in composition of a unstable atom
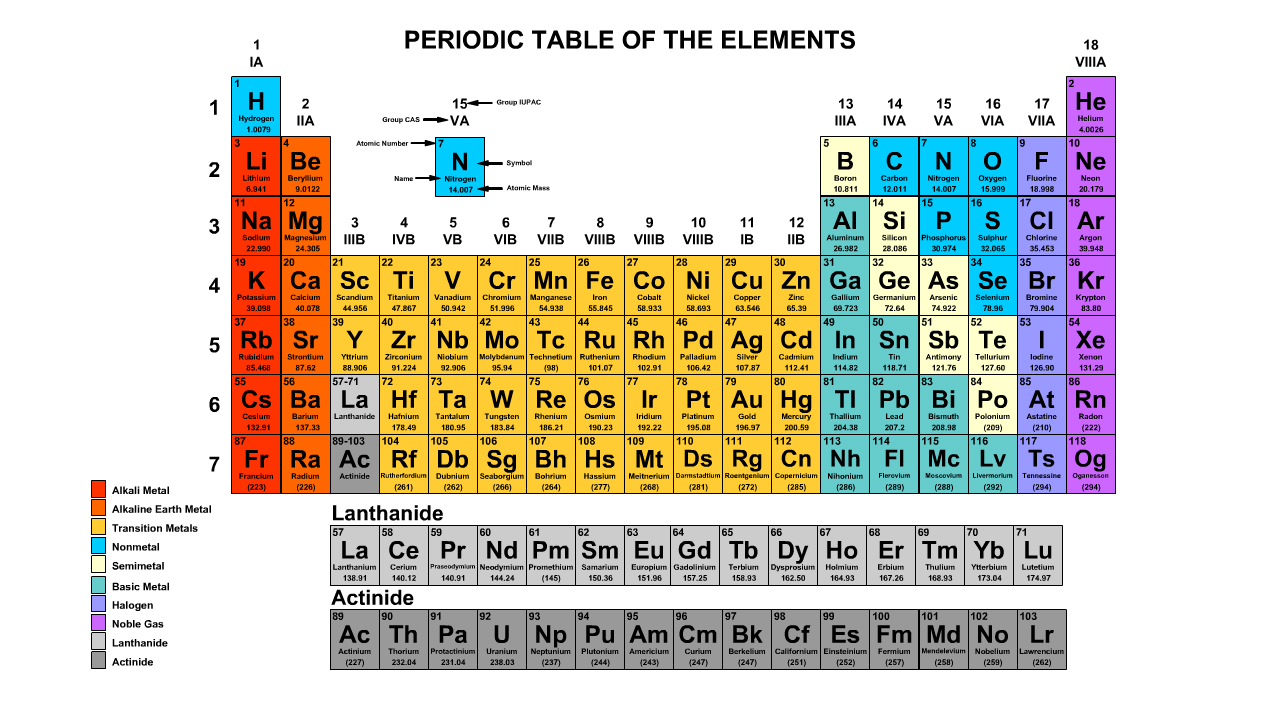
The Periodic Table
Reference a periodic table to determine the number of protons and electrons in a neutral element. The number of neutrons of a the most common isotope can also be calculated.
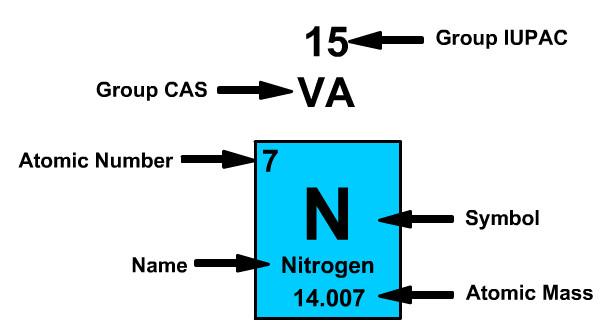
Atomic Number
The atomic number refers to the number of protons of the element. Periodic table cells have a smaller whole number and a larger number. The smaller whole number will be the atomic number.
Nitrogen has an Atomic Number of Seven
- Nitrogen always has seven positively charged protons.
- Neutral nitrogen has seven negative electrons.
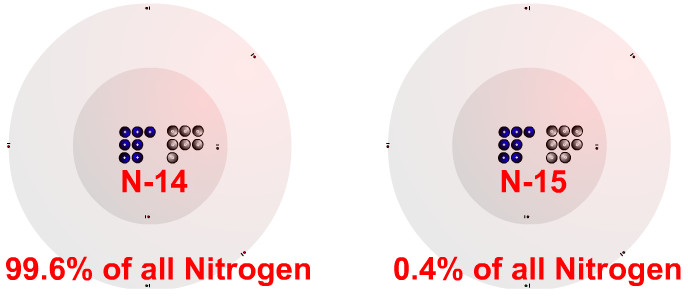
Isotopes and Nucleons
- Isotopes have the same number of protons but different numbers of neutrons. Round the atomic mass to the closest whole number to find the most common isotope.
- Nucleons is a collective term for the protons and neutrons in the nucleus of an atom. The number after an elements name or symbol represents the nucleons. N-14 has 14 nucleons.
Atomic Mass
The atomic mass refers to the number of protons and neutrons in an average isotope of the element.
Nitrogen on the Periodic Table has an Atomic Mass of 14.007
- The Nitrogen-14 isotope makes up 99.6% of Nitrogen found in nature.
- The Nitrogen-15 isotope makes up 0.4% of Nitrogen found in nature.
- 14.007 is the average atomic mass of all natural Nitrogen Isotopes. 14.007 is so close to 14 because so much of naturally occurring nitrogen is Nitrogen-14.
To determine the number of neutrons of an element
Example: Radon-220
- Find Radon (Atomic Symbol Rn) on the periodic table. The smaller number is the atomic number, representing the number of protons. Radon has an atomic number of 86 and therefore 86 protons.
- Ignore the atomic mass off the periodic table since this is the atomic mass of the average Radon found in nature. We specifically have one isotope, Radon-220, here.
- Take the number behind the element (220), which represents the number of nucleons of this specific radon isotope, and subtract the number of protons from it. Your solution here represents the number of neutrons in Radon-220.
- nucleons - protons = neutrons
- 220 nucleons - 86 protons = 134 neutrons
Example Problems
1. How many protons does nitrogen-15 have?
2. How many neutrons does nitrogen-15 have?
3. How many neutrons does nitrogen-13 have?
4. How many protons does Uranium-238 have?
5. How many neutrons does Uranium-238 have?
Links
- On to Nuclear Decay
- Back to the Main Nuclear Physics Page
- Back to the Stickman Physics Home Page
- Check out our StickMan Physics YouTube Channel
- Print a Periodic Table
- Equation Sheet